Gilead Sciences Korea highlights clinical and real-world data supporting earlier Yescarta treatment
As treatment lines increase, overall survival for diffuse large B-cell lymphoma (DLBCL) patients declines, underscoring the need for earlier application of CAR-T therapy. Gilead Sciences Korea held a media session on November 20th to discuss the clinical benefits of its CAR-T therapy Yescarta (axicabtagene ciloleucel).

Kijoon Min, Professor of Hematology at Seoul St. Mary’s Hospital, explained that lymphomas are broadly classified into Hodgkin and non-Hodgkin types, with non-Hodgkin lymphoma accounting for about 95% of cases. DLBCL represents roughly 30% of all lymphomas and is an aggressive subtype with a five-year survival rate of just 55.4%. It occurs more frequently in men aged 63 and older, and symptoms—such as lymph node enlargement, fever, and night sweats—often appear late, especially when tumors develop in the abdominal cavity.
After first-line therapy, 40% of DLBCL patients relapse or show poor outcomes. Among those who relapse, approximately 74% fail to respond to subsequent treatment, and overall survival drops below seven months.
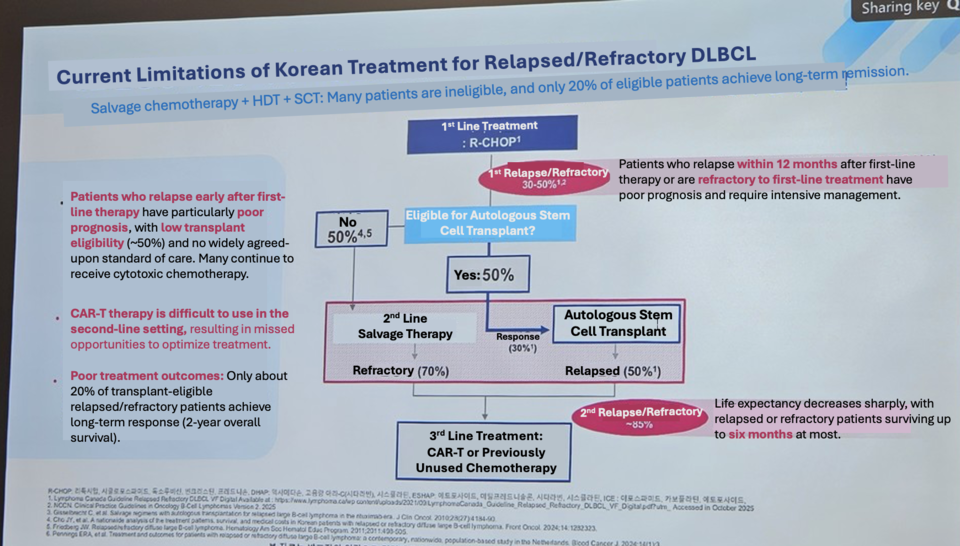
Prof. Min noted that the traditional option, allogeneic hematopoietic stem cell transplantation, presents significant limitations: outcomes worsen when transplanted in patients with already reduced remission rates, and nearly all patients except around 15% experience graft-versus-host disease. High-intensity conditioning is also difficult for patients aged 65 or older, creating a substantial unmet need. Due to these challenges, global guidelines recommend CAR-T therapy for patients who relapse within one year.
Yescarta received Korean approval in August for two indications: (1) adult DLBCL patients who relapse or are refractory within 12 months after first-line chemoimmunotherapy, and (2) adults with relapsed or refractory DLBCL or primary mediastinal large B-cell lymphoma after at least two lines of systemic therapy.
In a Phase 3 trial comparing Yescarta with standard therapy after first-line failure, Yescarta achieved an event-free survival (EFS) of 10.8 months—an 8.5-month improvement over standard therapy (2.3 months). Median overall survival (OS) was not reached in the Yescarta arm. The objective response rate (ORR) was 1.66 times higher than standard therapy, with no new neurotoxicity reported. In a separate study in patients who relapsed after second-line treatment, Yescarta delivered an OS of 42.6% and an EFS of 30.3%.
Prof. Min emphasized that rapid manufacturing enables timely infusion: “Because leukapheresis and T-cell collection can proceed directly to production, treatment can be delivered quickly, helping stabilize patients earlier.”
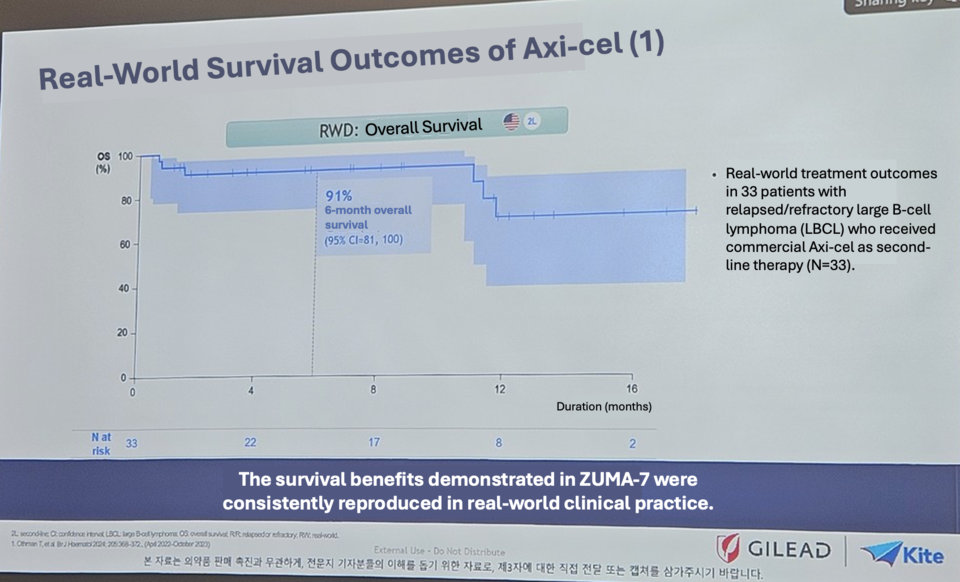
Sung-eun Kim, Medical Affairs Director, presented real-world data from 446 patients treated with Yescarta, showing a six-month OS of 91% and a one-year OS of 80%. “Survival is higher when CAR-T therapy is used earlier in the treatment course,” she said. “We aim to begin supply in the first half of next year and will work to ensure patients receive therapy as quickly as possible.”

