Vice President Kim outlines market outlook, growth drivers, and expansion strategy at 2025 Global Bio Conference

Samsung Biologics credited its rapid rise to the world’s top tier of contract development and manufacturing organizations (CDMOs) to a “speed management” and “standardization strategy.” Vice President Dong-jung Kim shared the company’s roadmap during his keynote at the 2025 Global Bio Conference on September 3.
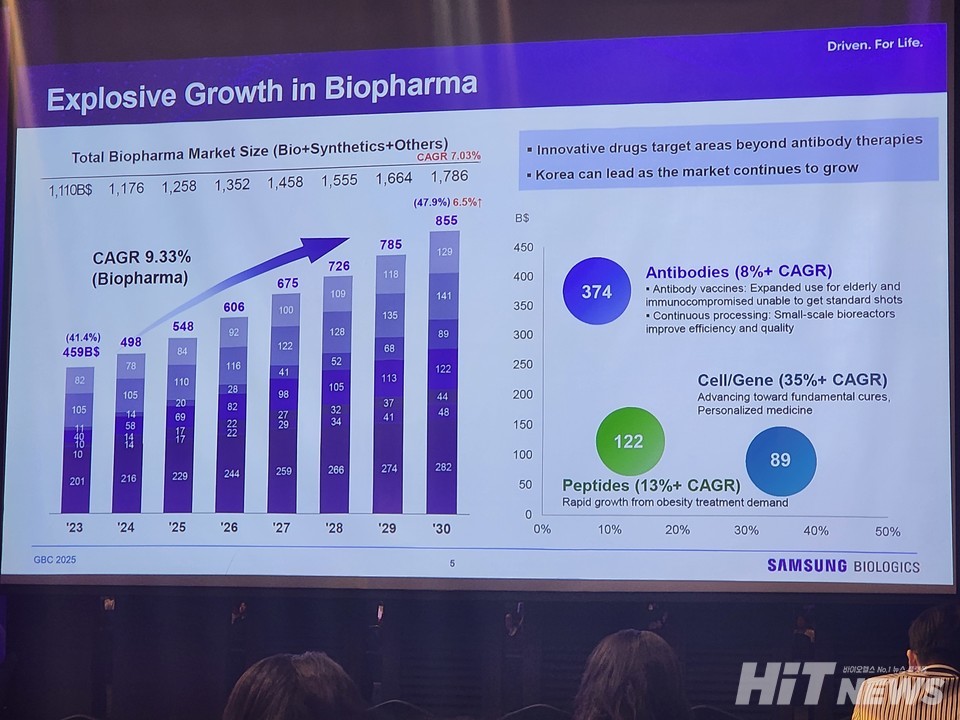
Kim pointed to biologics as the core driver of pharmaceutical industry growth. “The global pharmaceutical market reached $1.11 trillion in 2024 and is expanding at 7% annually. Biologics alone are growing at 9% a year, from $495 billion in 2024 to an estimated $786 billion by 2030,” he said.
Highlighting steady innovation, Kim noted antibody drugs remain robust, while cell and gene therapies are forecast to grow over 35% and peptides over 10%. “This is why we have never paused investment. CDMO demand will only continue to rise,” he stressed.
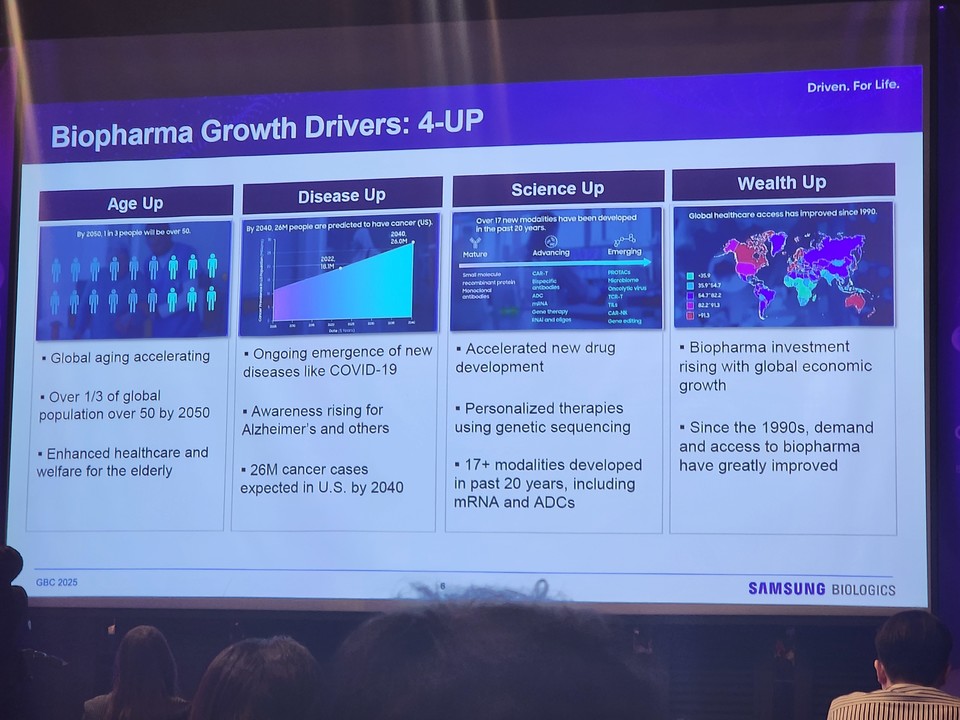
He summarized the market’s expansion under four drivers: Age Up, Disease Up, Science Up, and Wealth Up. Aging populations, rising cancer incidence—projected to add 26 million patients annually—emerging diseases, scientific advances such as mRNA modalities, and steady economic growth all contribute to rising biologics consumption.
Samsung Biologics’ strategy, Kim explained, was grounded in pragmatic analysis. Unlike new drug development—which typically takes a decade and $1 billion—Samsung leveraged its manufacturing expertise to pursue the CDMO model. “For Korean companies, assuming such high risks for uncertain returns is not feasible. That is why we chose the CDMO path,” he said.
The company applied Samsung’s core strength: speed in manufacturing. “Other firms take about eight years from plant construction to commercialization. Samsung can compress that timeline with unmatched efficiency,” Kim said.
Samsung Biologics broke ground on its first plant in May 2011, achieved GMP certification by 2013, and secured FDA cGMP approval by late 2015, generating revenue the following year. Successive plants at Songdo followed in rapid order.
The company accelerated further through “facility standardization,” applying a cookie-cutter approach to replicate plants with speed and consistency. “Plant 5 was completed in April 2024 in just two years—faster than the 30 months required for each of Plants 1 to 4,” Kim said. “The same strategy will cut timelines for Plants 6, 7, and 8.”
Within a decade of entering the business, Samsung Biologics secured contracts with 17 of the world’s top 20 pharmaceutical firms and posted $3.27 billion in revenue in 2023, cementing its status as a global CDMO leader.
Looking ahead, Kim said the company will expand beyond monoclonal antibodies to antibody-drug conjugates (ADCs) and adeno-associated virus (AAV) vectors. It has also entered the CRO business with organoid-based drug screening. “We will continue to explore new fields whenever our clients demand it,” he concluded.

