HitNews Special Report: The Cradle for Nurturing New Drugs Disappears, TIPS Payment Suspension Crisis
The recent suspension of TIPS funding, crucial for the survival of healthcare ventures involved in new drug development, AI solutions, and medical devices, has left companies in a difficult situation. The abrupt removal of support, which covers expenses from salaries to testing and consulting, has sparked growing criticism. Companies and institutions are expressing frustration, with no immediate solutions in sight.
What is TIPS? The Reason Behind Companies’ Struggle as Support Halts Midway
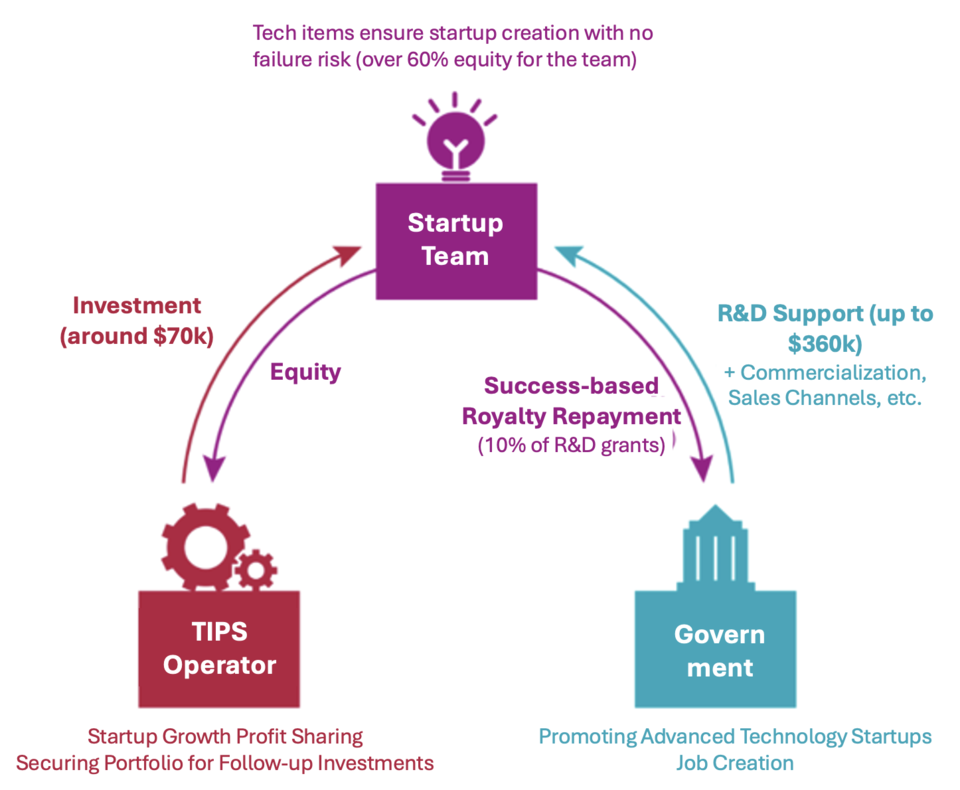
The Tech Incubator Program for Startup (TIPS) is a private investment-led technology startup support program under the Ministry of SMEs and Startups. It is divided into three categories: General TIPS, Scale-up TIPS, and Deep Tech TIPS. Although each program has different evaluation and support methods, they all aim to accelerate promising startups with technological potential. General TIPS provides $350,000 to $500,000 over two years, while Deep Tech TIPS can offer up to $1.2 million, making them crucial for early-stage healthcare ventures. General TIPS, predominantly for startups less than three years old, often serves as a benchmark for assessing a company's value and investment potential, as it indicates recognized technological merit.
The programs affected by the recent funding suspension are General TIPS and Deep Tech TIPS. However, companies in the Deep Tech TIPS category that were delayed in the selection process were spared. The issue with TIPS funding first surfaced early this year when the Ministry of SMEs and Startups announced a 20% reduction in support for companies selected for TIPS funding between 2022 and 2023, citing government R&D budget cuts. This caused concern in both the startup and investment communities, leading the Ministry to eventually commit to providing 100% of the promised funding.
Recently, the Ministry of SMEs and Startups delivered a shocking announcement to companies, stating that R&D budget payments related to TIPS funding would be suspended. The reason given was that the allocated budget for this year had already been spent. Companies were unaware of this situation, which created significant issues. The government has stated that the unpaid funds from this year will be distributed in 2025, but with no budget plan in place and uncertainty about the direction from the managing institutions, anxiety continues to grow.
“We Use Personal Funds to Continue” – Ventures Plead for Loan Access
Companies notified of the suspension of second-half funding are expressing that this news has hit them like a bolt from the blue. This situation is particularly challenging for firms in the biotech sector, where investment sentiment has recently cooled. The most pressing issue is securing funds to retain research talent.
The CEO of a biotech firm, which received Deep Tech TIPS support, describes the situation as follows: "TIPS primarily targets early-stage companies established within the last three years. Recently, with investments in biotech companies having stalled, most early-stage firms rely heavily on TIPS for salaries and research expenses. Most of the funds are used for staff salaries, and our company is no exception; the majority of our employees' salaries come from TIPS funds. If TIPS funding is suspended, we will face immediate difficulties in paying salaries from this month onwards."
"While the scale of TIPS funding is generally small, for startups that have not yet secured sufficient investment, it directly affects not only the new drug development schedule but also the survival of the company."
"The only solution is bank loans. If the government lacks funds, they should at least facilitate access to bank loans. Some companies selected for Deep Tech TIPS have not received their project funds. There is no information on when the funds will be provided, creating a comprehensive issue. We also need to sign the second-year agreement for Deep Tech TIPS, but we have not received any communication on when the agreement will be made or when the project funds will be disbursed, causing great anxiety. As a CEO of a company selected for Deep Tech TIPS, if the project funds are reduced or not provided, I will be unable to pay my employees' salaries, and this uncertainty is extremely stressful."

This issue is a simultaneous concern for multiple companies. Researchers in the healthcare sector typically command high salaries, and some companies even hire post-doctorate students of professors who have founded startups to maintain research continuity. Without access to loans or personal funds, there is a risk of losing valuable research talent.
The same applies to General TIPS companies. A company, which is developing a new drug, is facing difficulties due to the funding suspension. The new drug being developed by this company is in one of the hottest fields in the market right now. With major global pharmaceutical companies and ventures already competing, time is crucial to the success of the development process. The suspension significantly increases the risk of delays. The CEO of this company, recently notified and now deeply concerned, responded to the question of how to solve the problem as follows:
"Currently, our executives are using their personal funds to continue the trials. We can't afford to halt ongoing trials. Developing a new drug with this mechanism takes about 11 to 12 months to yield results. While it depends on the relationship, if the developing company cannot pay the test fees for six months, the testing company might be forced to stop the trials midway. For companies that haven't secured initial investments, the lack of TIPS support can be a significant blow."
The CEO of the above company emphasizes that delays in drug development due to the suspension of TIPS support can impact the sentiment of future investors during the initial investment stage.
"If drug development stalls, venture capitalists and other investors might raise concerns about the development speed. Speed is crucial; it allows the company to quickly present its progress to potential investors. Any issues arising from the suspension of TIPS could negatively affect future fundraising efforts from venture capitalists."
The CEO of another TIPS-supported company also predicts that the funding suspension will severely impact early-stage companies currently in the preclinical phase.
"The scale of government support is crucial for early-stage new drug development projects, making it a significant blow to early-stage companies. Fortunately, beyond the preclinical stage, where new drug development costs increase significantly, the impact may not be as severe. However, if there is an impact, increasing the investment amount will not be easy."
If salaries lag and new drug development slows, it could exacerbate the existing problem of talent drain in the biotech sector and further hinder new drug development speed compared to overseas competitors. Particularly, with two-thirds of the TIPS-supported companies being in healthcare, concerns arise that this suspension is like removing their life support.
Additionally, the operation of TIPS funds is critical not just for developing new drugs or products but also for important intellectual property and licensing issues. Beyond salary concerns, when developing technology or new drug substances, it is crucial to set the scope for patent applications, registrations, and future licensing. With many developing similar technologies, consulting to protect proprietary technology is essential. A significant portion of indirect costs covered by TIPS funds is often used for such consulting services, according to industry sources.
This suspension not only dries up the seeds of new drugs but also the ground that could potentially be used for recovery if anything goes wrong. This is highlighted by a CEO of a biotech that received Deep Tech TIPS funding."While TIPS generally provides small-scale support, for startups that have not yet secured sufficient investment, it directly affects not only the new drug development schedule but also the survival of the company."
Institutions Acknowledge Challenges: “Few Solutions, Much Frustration”
Due to this situation, even institutions managing TIPS funds are feeling the pressure. One such organization has already been receiving complaints from companies. A representative from this institution remarked, "What was expected has come."
The representative continued, "We have been aware since the beginning of the year that there might be difficulties in disbursing funds, so it seems like the inevitable has happened. Many TIPS-selected companies are expressing their disappointment and asking if things will improve next year."
"Companies are primarily demanding an environment where they can focus on research and development, trusting in government projects. However, the current trend in support policies is not favorable and is difficult to predict. We also find it frustrating that we cannot give clear answers to the selected companies."
The representative noted that this concern is not isolated to just one institution. "In fact, there was a meeting today involving related organizations. Everyone agreed that the suspension of TIPS support would be a significant issue moving forward. I'm sure other organizations also had a lot to discuss. We need to consider future directions and responses in discussions with the Angel Funding Association and other related parties."
Meanwhile, within the industry, there is significant concern about the impact on investments related to new drug development. Hit News plans to provide additional reports gathering opinions from fund managing institutions and investors on this matter.

