Genome & Company has successfully licensed out its 'GENA-111' to Debiopharm in a deal valued at $426 million. We prepared an in-depth analysis upon hearing the news.
Genome & Company, previously specializing in microbiome, announced last year its strategic shift towards oncology and ADC (Antibody-Drug Conjugate) expertise. This pivot raised eyebrows both internally and externally, with many questioning the viability of such a transition from microbiome to a completely different field.
However, by securing this licensing deal, Genome & Company has dispelled these doubts and introduced a fresh perspective on new drug development strategies. The key takeaways include: 1) the necessity of early licensing, 2) the potential for 'partial' ADC development, and 3) the critical importance of substance discovery platforms. Curious for more details? <Hit News> breaks it down for you in simple terms.
A Journey Back to the Birth of GENA-111
Let's rewind to the period before GENA-111 came into existence. At that time, Genome & Company was deeply engaged in analyzing tissues from cancer patients. Through its proprietary genomic analysis platform, GNOCLE, the company was comparing the genomes of cancer cells and normal cells, searching for elements that are highly expressed in cancer cells but minimally in normal cells.
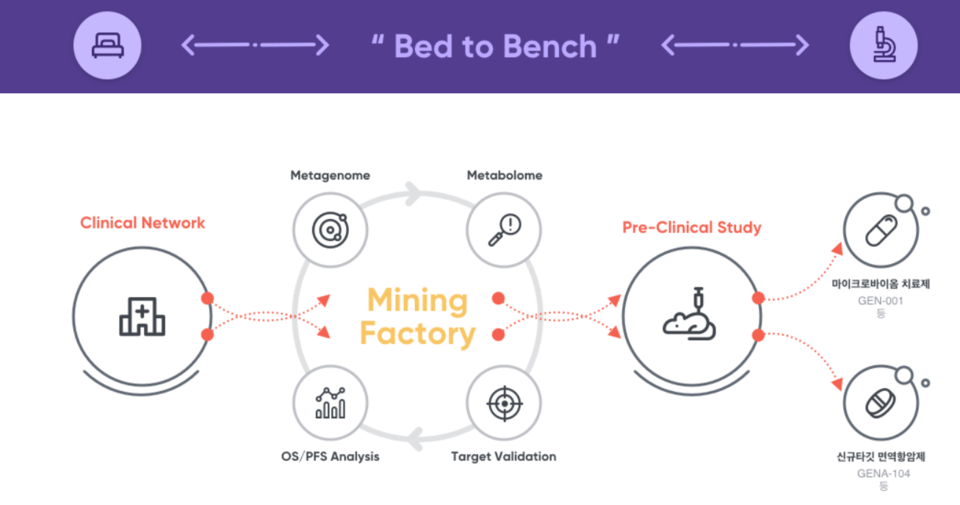
The goal of this endeavor was to discover new targets for anti-cancer drugs. The aim was to develop an antibody that would specifically target cancer cells while sparing normal cells. During this genomic analysis, they made a significant discovery. They identified a target('Target X') that was highly expressed in gynecological disease and hormone related cancer cells compared to normal cells.
Target X is essentially a substance that protrudes from the surface of cancer cells like an antenna. Besides its high expression rate, it has another intriguing characteristic: when it receives a specific signal or comes into contact with a particular substance, it gets internalized into the cell. This process is scientifically known as receptor-mediated endocytosis.
This property makes it particularly suitable for ADC (Antibody-Drug Conjugate) development. Imagine creating an antibody that binds to Target X and induces receptor-mediated endocytosis, then linking this antibody to a toxin via a linker to form an ADC. When administered to a cancer patient, the ADC would bind to the cancer cell, get internalized, and release the toxin inside the cell, thereby effectively killing the cancer cell.
How it all Started: A Collab with Debiopharm
Debiopharm was already a well-established pharmaceutical company dedicated to ADC development, equipped with its proprietary linker-payload platform known as 'Multilink.' This platform is versatile, allowing the attachment of various payloads and compatibility with multiple conjugation techniques.

However, having a linker and payload alone does not suffice for creating an effective ADC. A critical component is an antibody that can specifically target and bind to cancer cells. Debiopharm was on the lookout for a new antibody to incorporate into its Multilink platform when it discovered GENA-111 from Genome & Company about three years ago, sparking great interest.
Over the past three years, the two companies have collaborated on joint research to validate the efficacy and safety of GENA-111 in preclinical stages. The efficacy of GENA-111 was quickly confirmed. Nevertheless, Debiopharm, aware of the potential safety issues inherent in the ADC modality, conducted extensive in vivo safety evaluations. They performed experiments across multiple species to account for any interspecies differences.
With the safety results proving satisfactory, this rigorous collaborative effort culminated in the successful licensing agreement for GENA-111.
$426mn Deal = Low Quality Drug?
The recent licensing agreement for GENA-111, valued at $426 million, includes an upfront payment of $5 million. Some may question whether the deal size is small, given past licensing deals worth over $750 million with upfront payments exceeding $75 million. However, the size of a licensing agreement does not necessarily reflect the quality of the drug. Low-quality drugs wouldn't secure a licensing deal in the first place. The deal size reflects the target market size, while the upfront payment indicates the current development stage and associated risks.

Let's estimate the target market for GENA-111 ADC. According to the World Health Organization (WHO), there are approximately 320,000 ovarian cancer patients worldwide. Given that 80% of patients relapse after platinum-based chemotherapy and 42% respond to ELAHERE, the calculation is 320,000 x 80% x (100% - 42%) = approximately 148,000 patients.
Not all 148,000 patients would receive GENA-111 ADC. Only those expressing target X, the target of GENA-111, would be eligible. Although there is no specific data on Target X's expression in ovarian cancer, studies show that about 70% of breast cancer cells express Target X. Assuming a conservative estimate that 70% of ovarian cancer cells also express Target X, the number of eligible patients is 148,000 x 70% = approximately 100,000. Considering a treatment compliance rate of 70%, about 70,000 patients might actually receive GENA-111 ADC.
To estimate potential revenue, if we assume GENA-111 ADC is priced similarly to ELAHERE at $18,000 per treatment and consider 70,000 patients over a 10-year period, the total revenue could be $12.6 billion. However, this assumes successful market penetration in all countries and hospitals, which is unlikely.
Debiopharm, with around 400 employees, is primarily active in Europe. Therefore, the immediate market size might be about one-tenth of the global estimate, roughly $1.23 billion. Typically, the original patent owner receives about 60% of the revenue, but since Genome & Company only developed the antibody, not the entire ADC, a 30% share is more reasonable. Thirty percent of $1.26 billion is approximately $370 million, which aligns closely with the actual deal value of $426 million. Thus, Genome & Company's licensing deal appears quite reasonable.
Regarding the upfront payment, it tends to be smaller when the drug is in the early development stages. This early stage justifies the relatively low upfront payment of $5 million.
The Meaning of GENA-111 L/O for Our Industry
Genome & Company's recent licensing agreement offers three significant insights: 1) despite being a First-in-Class substance, it was licensed out at a very early stage, 2) even a novel antibody, rather than a complete ADC, can secure a licensing deal, and 3) the pivotal role of the GNOCLE platform in making this possible.
First-in-Class drugs typically carry high development risks, making licensing deals challenging before clinical data is available. However, GENA-111 successfully secured licensing at a very early stage. This success is likely due to targeting a relatively lower-risk modality. ADCs have undergone numerous proof-of-concept validations and have accumulated real-world data, making them less risky compared to other novel modalities.
Additionally, GENA-111 is just an antibody, a part of an ADC, rather than a complete ADC. This fact introduces a new development strategy for the industry. Instead of investing significantly higher resources into developing a complete ADC, companies can focus on developing the antibody component within their means and seek licensing opportunities.

