Revisiting BIO USA 2024
As the curtain falls on BIO International 2024 (BIO USA), we take a moment to reflect on the journey and achievements of Korean pharmaceutical and biotech companies at this year's convention in San Diego.
Hit News focused on interviewing representatives from various companies to delve into their international strategies. We aim to highlight notable strategies and collaborate on refining those that need improvement. To all the K-Bio colleagues who joined us on this four-day journey, your hard work is greatly appreciated.
Celltrion’s Unique Approach at BIO USA 2024

Unlike most Korean companies that set up booths to introduce their CDMO (Contract Development and Manufacturing Organization) businesses, Celltrion focused on promoting its brand with a large-scale booth. In addition to using the meeting rooms provided by the organizers, Celltrion had its own meeting rooms.
BIO USA is not primarily a booth-centric event. The most important business partnering discussions take place in separate meeting spaces. However, companies still set up booths at BIO USA to promote CDMO, CRO (Contract Research Organization) services, and materials, parts, and equipment (MPE).
Celltrion's main business areas are not CDMO, CRO, or MPE. As is well known, their primary business item is biosimilars. This focus on biosimilars drove Celltrion to engage in large-scale promotion at BIO USA.
Despite the pharmaceutical and biotech industry being driven by objective and empirical data, it is also influenced by human sentiment. Physicians tend to prescribe medications they are more familiar with, and pharmacists recommend drugs that are well-known to them. This also applies to the business development (BD) teams of global pharmaceutical and biotech companies, the main attendees of BIO USA.
Celltrion has already launched several biosimilars internationally, including 'Remsima', 'Remsima SC', 'Truxima', 'Herzuma', 'Vegzelma', 'Yuflyma', and 'Zymfentra'. Biosimilars of Stelara, Xolair, Eylea, Prolia, and Actemra are also expected to be approved within the year. Commercialized products need additional licensing and supply agreements for different regions, while those still in development will need these agreements post-commercialization.
BIO USA is the event where BD teams, who handle these licensing and supply agreements, gather in large numbers. For them, a company's brand image is significant when considering biosimilar products. With multiple biosimilars of similar price and quality available, familiarity with a company's name often influences their decision.
Brand image is just one of many factors influencing their decisions, but it certainly holds weight. Therefore, Celltrion conducted customer surveys at their booth. The surveys, consisting of about ten questions, asked visitors if they had heard of Celltrion and what they thought of its products. These surveys were conducted via tablets, and visitors who completed them received LEGO-style souvenirs. This strategy allowed Celltrion to both promote its brand and gather customer data, achieving two goals with one effort.
Hanmi Fine Chemical’s Peptides and mRNA Capping
The late Chairman Sung-Gi Lim of Hanmi Pharmaceutical had a keen interest in drug synthesis technology. Within Hanmi Group, drug development was managed by Hanmi Pharmaceutical, while Hanmi Fine Chemical handled drug synthesis. Initially, Hanmi Fine Chemical produced Hanmi Pharmaceutical's proprietary products. As their expertise grew, they branched into an independent business, leading to CEO Jong-Hoon Lim of Hanmi Science emphasizing new ventures like Contract Development and Manufacturing Organization (CDMO).
At BIO USA, Hanmi Fine Chemical promoted its peptide synthesis and mRNA capping technologies, which gained attention due to COVID-19.
Previously, the peptide CDMO market struggled due to the rise of mRNA drugs during the pandemic. Many biotechs preferred developing mRNA drugs, believing it better to inhibit disease-causing peptides at the mRNA stage. However, as the pandemic became endemic, interest in peptide drugs, especially for obesity and diabetes, resurged, while mRNA drug development consolidated among specialized companies. Hanmi Fine Chemical adapted its strategies accordingly.
For peptide synthesis, Hanmi Fine Chemical emphasized PEGylation technology to enhance structural stability and prolong efficacy. This long-standing strategy was highlighted at BIO USA, resulting in a significant increase in peptide synthesis inquiries compared to the previous year.
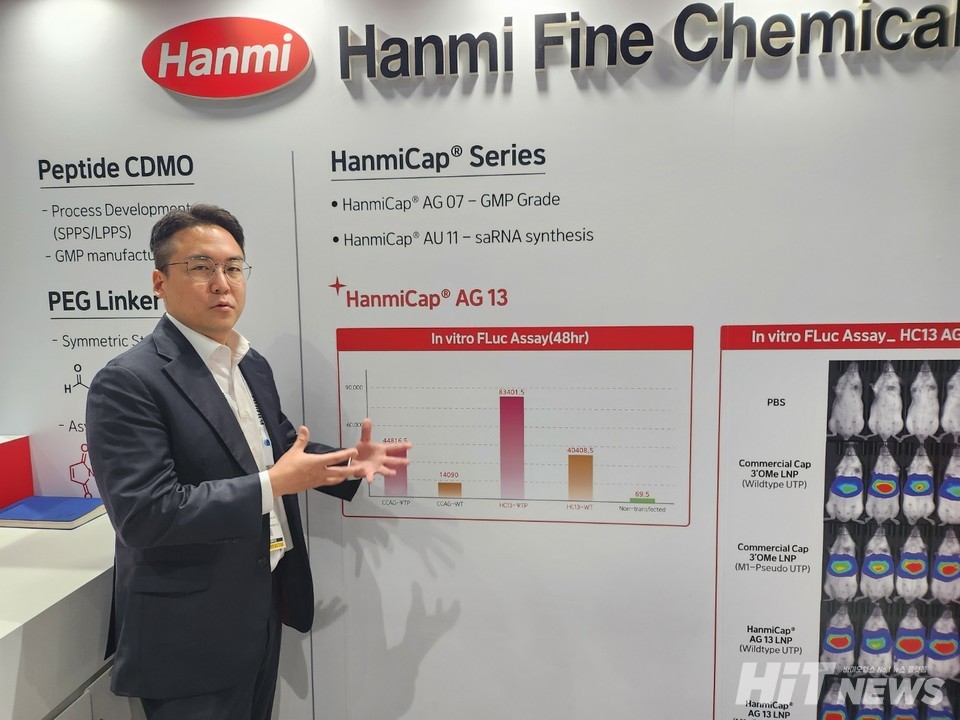
In mRNA capping, Hanmi Fine Chemical targeted its main competitor, T company, known for high pricing. HanmiCap, their mRNA capping brand, focused on price competitiveness and superior technology, offering higher transcription efficiency and lower double-stranded RNA content, reducing potential immune responses and side effects.
관련기사
- 바이오USA | '들어봤나' 셀트리온과 '역발상' 한미정밀화학
- 단독|중국 견제?...바이오 USA에 뜬 국가안보실 3차장
- |BIO USA 현장| 똑똑똑, 동물 당뇨신약 있나요? CNS 약물은요?
- 초점|지놈앤컴퍼니 GENA-111은 어떻게 L/O 성공했을까
- 4조 빅딜 더글라스 팸브로 "K-바이오텍, 나스닥으로 가라"
- 보도자료에 못 담았던 제약바이오협회의 바이오USA 후일담
- C'D'MO 집중 차바이오... MD앤더슨의 '원픽' 브이에스팜텍, 왜?
- How Did Genome&Company Successfully L/O GENA-111?
- Douglas Fambrough Says, “K-Biotech Should Go to NASDAQ”

