Telminuvo 20/1.25 mg Launch Press Briefing
“Strong BP Reduction at Low Dose” — Targeting Young Patients’ Need for a Low-Burden Starting Therapy

Chong Kun Dang has launched Telminuvo 20/1.25 mg, a low-dose combination therapy designed for early hypertension management. Clinicians at the launch event emphasized that younger patients often show lower treatment initiation and adherence, and that a low-burden starting dose may help minimize barriers to starting therapy.
On November 21, the company held a press briefing at the Josun Palace Hotel in Seoul, where cardiovascular experts—including Professor Jin-Ho Shin of Hanyang University Hospital and Professor Dae-Hee Kim of Asan Medical Center—discussed evolving treatment guidelines and supporting clinical evidence.
Professor Shin, who chaired the session, noted that major hypertension guidelines such as ESG and JSH increasingly recommend dual therapy as the preferred initial approach. “Low-dose combination therapy helps minimize concerns about side effects while enabling patients to reach target blood pressure more efficiently,” he said.

Professor Dae-Hee Kim highlighted significant shifts in global treatment standards over the past decade. “Both U.S. and European guidelines now recommend more aggressive blood pressure control from the start, with earlier achievement of a target below 130/80 mmHg,” he said. “In this context, low-dose combination therapy offers stronger clinical justification, providing faster and more stable control than monotherapy.”
He added that stepwise dose escalation with monotherapy—traditionally the standard pathway—faces limitations in real-world practice, leading to increasing treatment complexity, lower adherence, and reduced success in achieving target blood pressure.
According to the Korean Society of Hypertension’s Fact Sheet, Korea’s hypertension control rate has surpassed 60% for the first time, yet fewer than 30% of high-risk cardiovascular patients achieve the recommended target of below 130/80 mmHg.
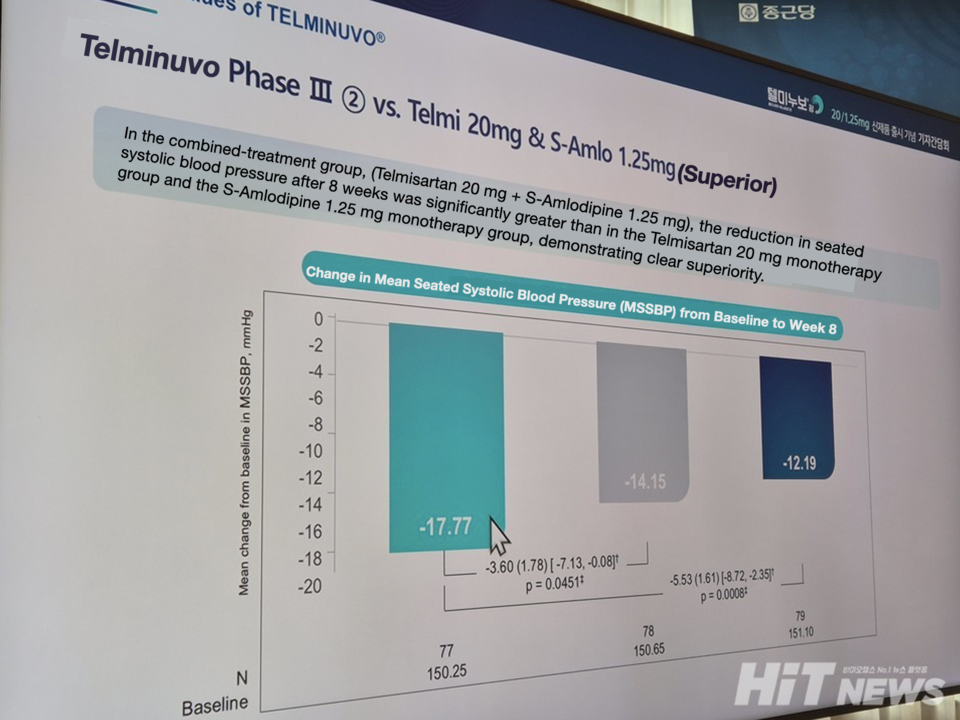
Phase 3 clinical results presented at the event further underscored the advantages of the low-dose combination. Over eight weeks, Telminuvo 20/1.25 mg achieved greater reductions in blood pressure than standard monotherapy and showed consistently higher target-BP attainment. Compared with telmisartan or S-amlodipine alone, the combination produced the largest overall drop in blood pressure.
Professor Kim stressed that improving adherence—particularly in younger patient groups—is critical to raising national control rates. “The more pills a patient takes, the lower the adherence,” he said. “When medication count increases from three to four, adherence drops by about 38%, and from four to five, by roughly 72%.”
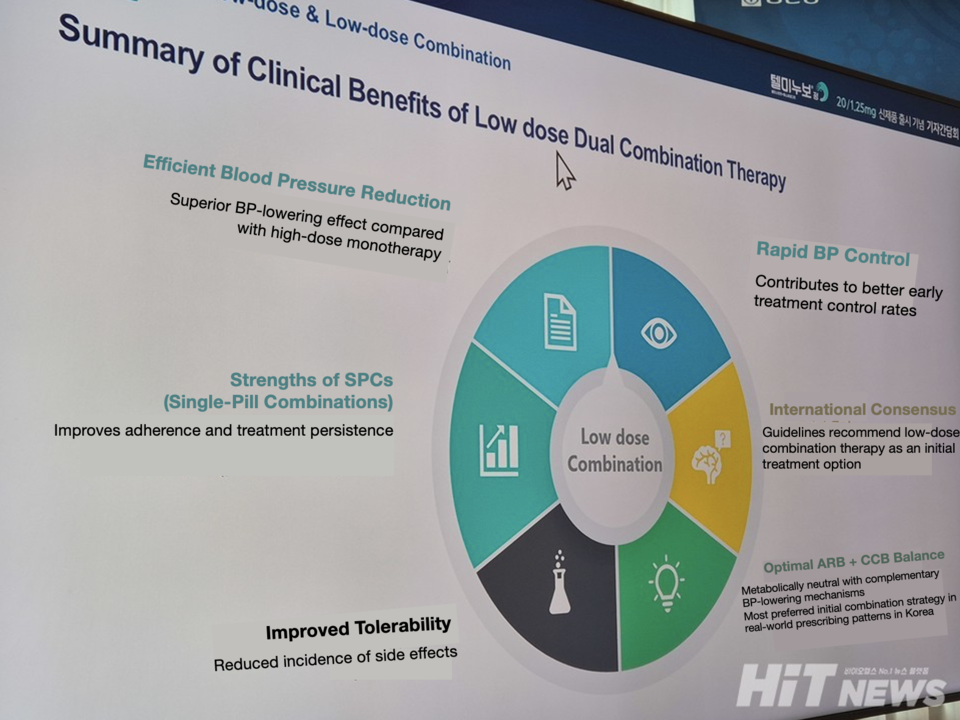
With the new launch, Chong Kun Dang has expanded the Telminuvo portfolio to six dosage strengths. Sumi Yoon, Director of Marketing, noted that patients with chronic diseases often feel uneasy when their medication changes. “Starting therapy with Telminuvo allows patients to remain on the same brand even as doses are adjusted, improving adherence and treatment continuity,” she said.
She added that the new strength “is not merely an additional option but one that shifts the initial treatment strategy itself.” The company plans to continue strengthening the clinical value of its telmisartan-based portfolio and its overall market competitiveness by prioritizing patient-centered treatment solutions.

