Innovative Oral Therapy with Multi-Target Mechanism Redefines the Fight Against Alzheimer’s Disease
According to UK-based market research firm GlobalData, 18 late-stage drug candidates are projected to enter the Alzheimer’s disease (AD) treatment market between 2020 and 2030. This list includes therapies from prominent global pharmaceutical companies and innovative biotech firms. Among these, AriBio’s AR1001 has emerged as a standout candidate.
AR1001, alongside Annovis’ Buntanetap, has been identified as having the highest clinical potential. Both drugs, now in Phase 3 clinical trials, have achieved higher clinical efficacy scores than the existing treatment Leqembi. Notably, AR1001 also received the highest commercial evaluation score among non-big pharma candidates, solidifying its reputation as a promising new therapy.
Addressing Treatment Gaps: Oral Delivery and Multi-Target Mechanism
The current AD treatment market is largely dominated by antibody-based injectable therapies. While effective, these treatments face challenges such as high costs and the inconvenience of administration, which can hinder long-term patient adherence. AR1001, as an oral therapy, offers significant advantages in cost and ease of use, potentially improving patient outcomes.
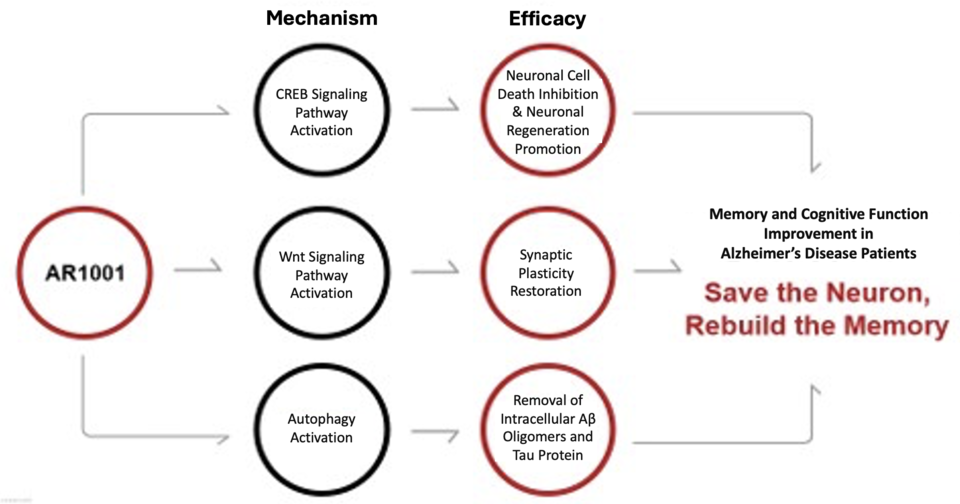
What sets AR1001 apart is its multi-target approach to combating AD. While many existing therapies focus on single biological pathways, AR1001’s mechanism simultaneously addresses multiple physiological processes. Classified as a phosphodiesterase-5 (PDE5) inhibitor, AR1001 demonstrates multi-functional therapeutic effects, offering a new perspective on treating Alzheimer’s disease.

The Science Behind AR1001: A Multi-Target Strategy
Imagine the brain as a house. Over time, it accumulates damage and debris, similar to a house becoming uninhabitable without regular maintenance. Alzheimer’s disease represents a similar state, with harmful proteins like Aβ and pTau accumulating in the brain.
Unlike traditional therapies that directly target these proteins, AR1001 addresses the underlying dysfunction. It inhibits PDE5, which is overactive in the temporal cortex—a critical area for memory and language—of AD patients. PDE5 breaks down cyclic guanosine monophosphate (cGMP), a vital signaling molecule. Overactivity of PDE5 in AD leads to reduced cGMP levels, disrupting brain cell communication and triggering cellular damage.
cGMP is central to the “NO-cGMP-CREB” signaling pathway, essential for neuronal survival and connectivity. By activating CREB, cGMP supports genes responsible for brain health. It also influences the Wnt signaling pathway through protein kinase G (PKG), which enhances neuron growth and inhibits GSK-3β, a protein involved in producing pTau. By preserving cGMP levels, AR1001 aims to restore these critical pathways, enabling brain repair and improved function.
Several PDE5 inhibitors, including sildenafil and tadalafil, are already FDA-approved for other conditions like pulmonary hypertension. Research has suggested sildenafil’s potential in treating AD. However, AR1001 demonstrates over ten times the inhibitory strength of sildenafil, positioning it as a next-generation therapeutic candidate.
By enhancing cGMP signaling, AR1001 not only supports neuronal survival and connectivity but also reactivates autophagy, the brain’s natural cleaning system. This comprehensive, multi-target mechanism underpins its potential as a groundbreaking therapy in Alzheimer’s treatment.
AriBio is conducting a global Phase 3 clinical trial of AR1001, involving 1,150 participants. The company anticipates completing patient dosing by the end of 2025 and plans to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in 2026.
With its innovative approach and strong clinical potential, AR1001 represents a significant step forward in addressing the unmet needs in Alzheimer’s disease treatment.

