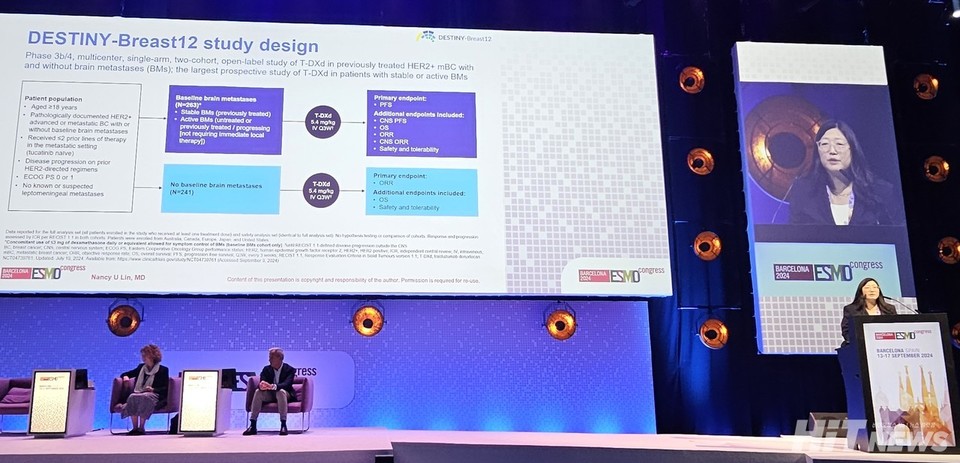
Phase 3b/4 'DESTINY-Breast12' Trial Targets Patients with Brain Metastases
Stable Brain Metastasis: 62.9% PFS, Active Brain Metastasis: 59.6% PFS, Showing Similar Results
AstraZeneca and Daiichi Sankyo’s antibody-drug conjugate (ADC) Enhertu (trastuzumab deruxtecan) has shown substantial benefits in progression-free survival (PFS) and overall survival (OS) for HER2-positive breast cancer patients with brain metastases.
On September 14th, at the ESMO Annual Meeting, the results from the Phase 3b/4 Destiny-Breast12 study were presented. This trial specifically focused on patients with HER2-positive metastatic breast cancer, particularly those with brain metastases. It marks the first multinational, multi-center, open-label Phase 3b/4 trial evaluating the efficacy and safety of Enhertu in 500 patients, both with and without brain metastases.

Dr. Nancy Lin, Head of the Breast Oncology Program at Dana-Farber Cancer Institute in Boston and Principal Investigator of the DESTINY-Breast12 trial, led the presentation. She highlighted that Enhertu demonstrated clinical effectiveness in HER2-positive breast cancer patients with brain metastases, aiming to confirm similar efficacy and safety, irrespective of brain metastasis.
The trial assigned 241 HER2-positive metastatic breast cancer patients without brain metastases to Cohort 1. Cohort 2 included 263 patients with active brain metastases, categorized into untreated patients not requiring immediate local treatment and those with progressing or stable brain metastases following prior treatment.
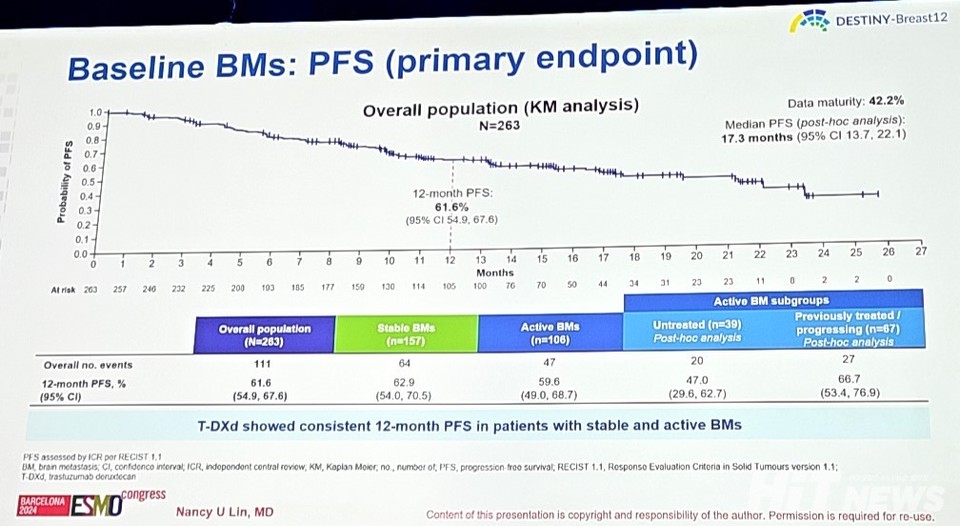
In Cohort 2, the primary endpoint—12-month PFS—was 61.6% (95% CI: 54.9-67.6). Patients with stable brain metastases had a PFS of 62.9% (95% CI: 54.0-70.5), while those with active brain metastases showed a comparable PFS of 59.6% (95% CI: 49.0-68.7).
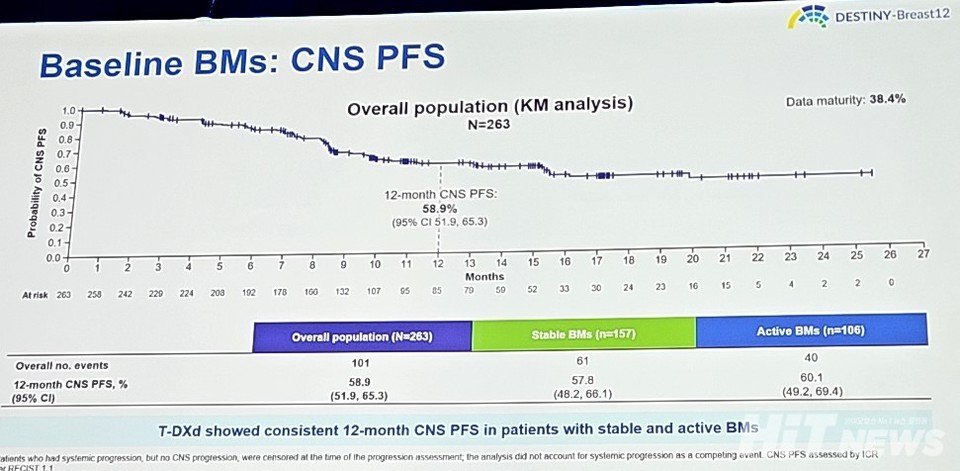
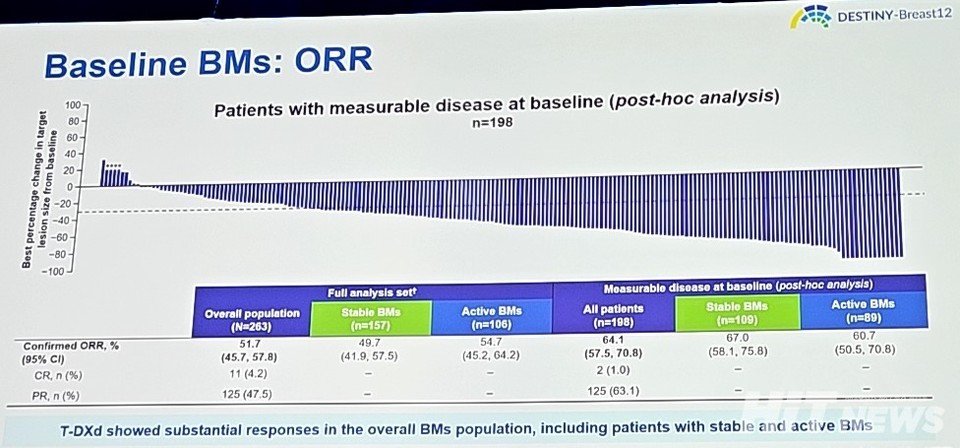
The secondary endpoint, 12-month CNS PFS (Central Nervous System Progression-Free Survival), was 58.9% (95% CI: 51.9-65.3), with results being similar across stable brain metastases at 57.8% (95% CI: 48.2-66.1) and active brain metastases at 60.1% (95% CI: 49.2-69.4). The overall response rate (ORR) was reported as 51.7% (95% CI: 45.7-57.8).
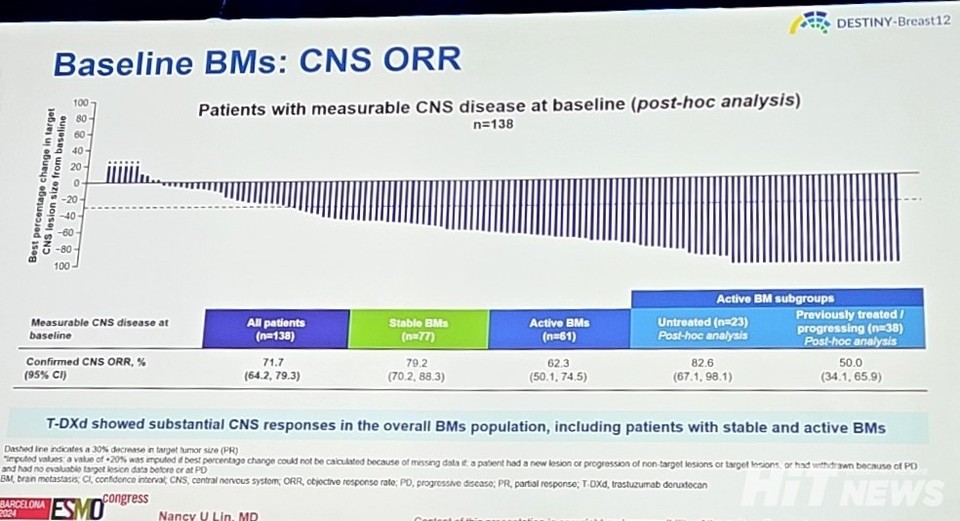
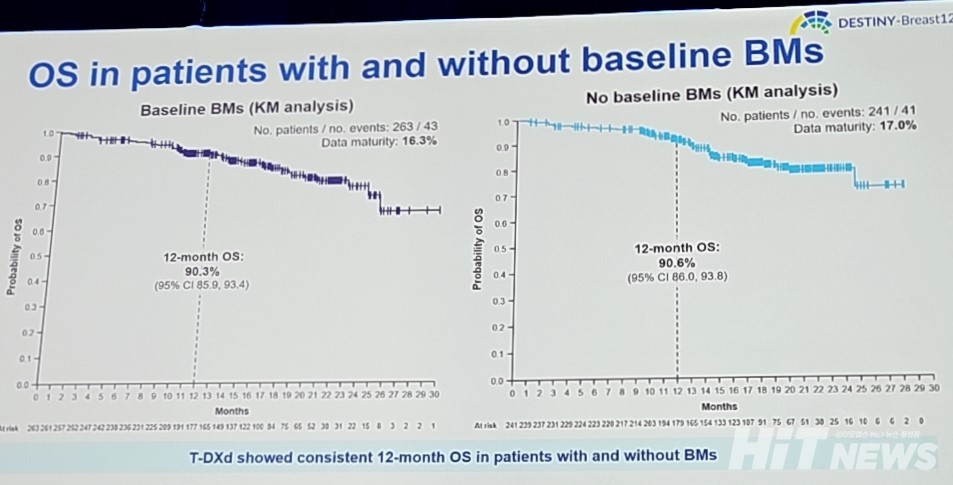
In Cohort 1, consisting of patients without brain metastases, the ORR was confirmed at 62.7% (95% CI: 56.5-68.8), with a complete response rate of 9.5% and a partial response rate of 53.1%. The 12-month OS was similar in both cohorts, with Cohort 1 at 90.3% (95% CI: 85.9-93.4) and Cohort 2 at 90.6% (95% CI: 86.0-93.8), though the data are still maturing.
The safety profile of Enhertu remained manageable across both cohorts, with no new concerns identified. Common side effects included nausea and fatigue, with neutropenia being the most frequent Grade 3 or higher adverse event.
Dr. Lin noted that the study reinforces Enhertu’s potential benefit for patients with stable or active brain metastases, though concerns related to interstitial lung disease (ILD) and pneumonitis remain and require ongoing monitoring.
Despite numerous treatment options for HER2-positive breast cancer, therapies that show significant clinical benefit for patients with brain metastases remain limited.
Enhertu had previously demonstrated significant therapeutic benefits in a subgroup analysis of 82 patients with brain metastases in the approved DESTINY-Breast03 trial. However, it is important to note that this study did not include patients with active brain metastases. In that subgroup analysis, the median progression-free survival (mPFS) for patients with brain metastases was 15.0 months (95% CI: 12.5-22.2) with Enhertu, compared to 3.0 months (95% CI: 2.8-5.8) in patients treated with *Kadcyla* (trastuzumab emtansine), resulting in a fivefold increase in the Enhertu group (HR=0.25, 95% CI: 0.13-0.45).
The intracranial objective response rate (IC-ORR) was 65.7% for Enhertu compared to 34.3% for Kadcyla. Additionally, the overall response rate was 67.4% for Enhertu versus 20.5% for Kadcyla.
Further, Enhertu displayed therapeutic benefits in the single-country ROSET-BM study and the single-center TUXEDO-1 study, both presented at the San Antonio Breast Cancer Symposium (SABCS 2023). Although the overall survival (OS) data remains immature, the TUXEDO-1 study showed an IC-ORR of 73.3% in patients with active brain metastases and a mPFS of 21 months in the final analysis.

