Dual Antibody Therapy Shows Promise in Clinical Trials

ABL Bio is expected to advance in the development of treatments for gastric and esophageal cancer through subsequent clinical trials of ABL111 (development code name), a dual-antibody immune oncology agent based on 4-1BB, in triple combination therapy and monotherapy.
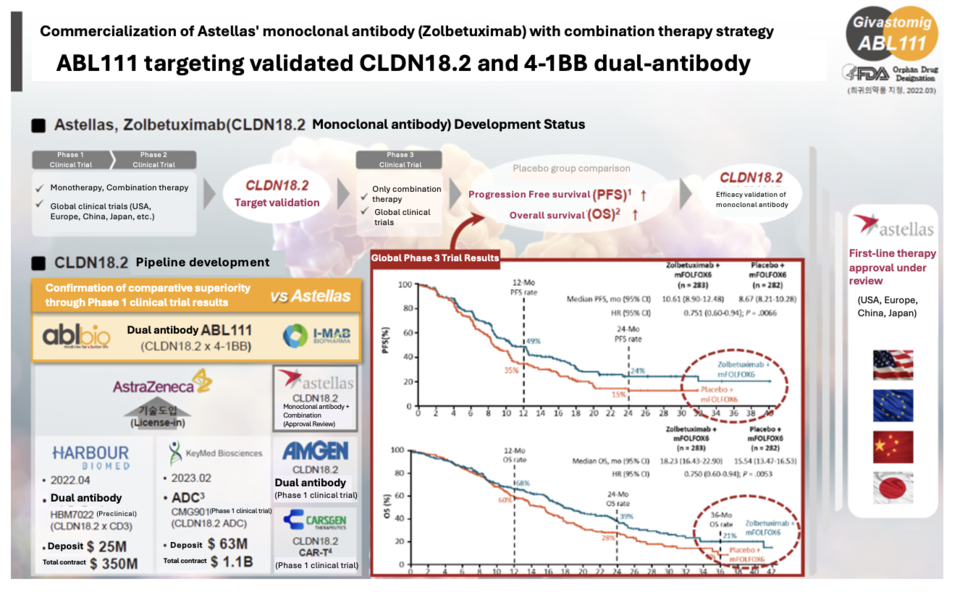
According to ABL Bio on March 20th, ABL111 is a dual-antibody targeting both Claudin18.2 and 4-1BB simultaneously. In animal experiments, ABL111 has demonstrated superior tumor suppression effects compared to Claudin18.2 alone or 4-1BB alone, and animals showing anticancer effects have suppressed recurrence of the same cancer cells through immune memory responses.
ABL111 is a dual-antibody pipeline jointly developed by ABL Bio and NASDAQ-listed company I-Mab. It aims to develop a drug with superior efficacy compared to AstraZeneca's gastric cancer treatment, Zolbetuximab.
ABL Bio aims to develop a best-in-class therapy within its portfolio using a triple combination strategy of 'ABL111+Chemotherapy+PD-(L)1'. The company explains that it considers combination therapy as a first-line treatment and contemplates the standalone administration of ABL111 as a third-line treatment.
ABL Bio CEO Sang-Hoon Lee stated during last year's October online Investor Relations (IR) meeting, "The clinical Phase 1 of ABL111 progressed through three stages. We completed the first stage, which involved 'dose escalation.' Subsequently, the company experimented with dose expansion at 5, 8, 12, and 15 mg/kg." He further revealed, "Through tumor expansion (the final stage), we are conducting clinical trials targeting gastric cancer in the United States and China at the optimal dose of 12 mg/kg."
According to the company, ABL111 demonstrated a high level of safety, with no Grade 4 or higher treatment-related adverse events (TRAE) that could be life-threatening identified among the 12 TRAEs occurring in over 5% of the total 55 patients participating in Phase 1 clinical trials. The company explained that based on interim results from Phase 1, ABL111 showed superior safety, efficacy, and tolerability compared to Zolbetuximab. Additionally, ABL111 exhibited stronger effects at effective doses and in various cancer types, demonstrating excellent pharmacokinetics.
An ABL Bio official stated, "ABL111 has received Orphan Drug Designation (ODD) as a treatment for gastric cancer (including gastroesophageal junction cancer) from the U.S. Food and Drug Administration (FDA)." They further explained, "Given the high expression of Claudin18.2 in gastric cancer and other related cancers, AbL Bio and I-Mab currently plan to develop ABL111 as a treatment for gastric and esophageal cancer. In the future, we aim to expand its use to other cancer types, such as pancreatic cancer, where Claudin18.2 is expressed."
Continuing, the official stated, "Last October, at the European Society for Medical Oncology (ESMO), the company presented interim results from Phase 1 clinical trials of ABL111. However, discussions with I-Mab are necessary regarding the timing of the release of the final Phase 1 data. Additionally, it was noted that there are no plans to announce the final Phase 1 data at ESMO 2024 in September."
Industry experts, including researcher Kyung-Tae Han, underscore ABL111's superior efficacy and optimized safety profile compared to conventional therapies. ABL Bio's strategic roadmap includes triple combination clinical trials as a primary treatment modality, signaling a paradigm shift in cancer therapeutics.

