From BBB Shuttles to Long-Acting Formulations, Korean Firms Advance CNS Therapeutics
Korean biotech companies are ramping up efforts in the central nervous system (CNS) therapeutics field, with a particular focus on Alzheimer’s disease. Traditionally focused on oncology and metabolic diseases, the industry is now pivoting toward the complexities of neurodegenerative disorders, leveraging a variety of mechanisms and advanced blood-brain barrier (BBB) delivery technologies to compete globally.
Alzheimer’s disease, marked by multifactorial pathophysiology—including amyloid-beta plaques, tau protein phosphorylation, synaptic loss, inflammation, and BBB dysfunction—has seen limited therapeutic progress despite recent U.S. FDA approvals targeting amyloid clearance. In response, Korean companies are diversifying treatment strategies with novel targets and innovative delivery platforms.
K-Bio Focuses on BBB: From 'Shuttles' to 'Stabilizers'
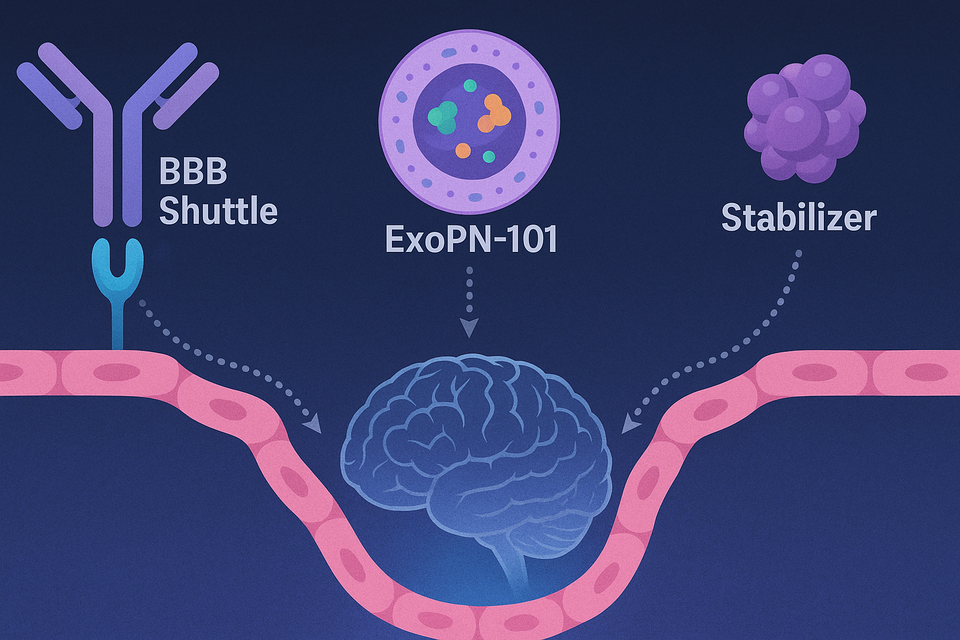
As the BBB poses a major hurdle to CNS drug delivery, Korean biotechs are developing platforms that shuttle drugs across the barrier or stabilize its integrity.
ABL Bio is advancing Grabody-B, a BBB shuttle platform targeting the insulin-like growth factor 1 receptor (IGF1R) to enable receptor-mediated transcytosis of antibodies into the brain. Preclinical studies in aged mouse models demonstrated sustained brain penetration, and the data will be unveiled at the 2025 Alzheimer’s Association International Conference (AAIC). ABL Bio’s licensing deal with GSK, signed in April and valued up to $2.9 billion, validates the platform’s commercial potential.
LIS Cure has developed ExoPN-101, an exosome-based delivery platform capable of transporting ASOs, siRNAs, and small molecules with high biocompatibility and reduced immunogenicity. In non-human primate studies, ExoPN-101 showed superior brain delivery compared to other shuttle technologies. The company is in active collaboration and licensing discussions with global pharmaceutical firms, backed by material transfer agreements (MTAs).
Curacle is taking a different approach with CU71, a candidate that stabilizes the BBB and restores cerebrovascular integrity. By enhancing tight junction proteins and reducing amyloid accumulation and inflammation, CU71 showed preclinical superiority over donepezil in memory restoration. Curacle has filed for international patent protection and will present the findings at AAIC 2025.
Formulation Innovation Heats Up: Focus on Long-Acting and Oral Therapies
With Alzheimer’s treatments requiring long-term dosing, Korean companies are prioritizing formulation innovation to improve patient adherence.
G2GBIO’s InnoLAMP platform enables high drug loading and extended-release delivery in a once-monthly subcutaneous injection. Its lead assets, GB-5001 and GB-5001A, completed Phase 1 trials in Canada and South Korea and aim to improve adherence by maintaining efficacy with a single monthly dose.
AriBio’s AR1001 is an oral small-molecule therapy that targets multiple Alzheimer’s pathways, including PDE5 inhibition and tau phosphorylation suppression. The drug showed cognitive improvement in a U.S. Phase 2 study and is currently in global Phase 3 trials across 13 countries. In advance of topline results expected in 2026, AriBio secured a $590 million licensing deal with a UAE sovereign fund affiliate, raising its total deal value to $1.4 billion.
Korea’s biotech sector is accelerating its push into Alzheimer’s therapeutics through differentiated approaches in BBB penetration and formulation technologies. As platforms mature and clinical milestones are met, global partnerships and trial outcomes will determine which firms can stake a lasting claim in the competitive CNS market.
관련기사
- K-바이오, 알츠하이머로 글로벌 도전... 조인트스템, 이번엔 허가?
- '암·비만 다음은 뇌'… 알츠하이머에 몰리는 K-바이오
- 큐라클, 알츠하이머병 치료제 후보 'CU71' PCT 국제특허 출원
- 큐라클, 알츠하이머치료 후보물질 CU71 전임상 결과 발표
- 에이비엘바이오, AAIC 2025서 BBB 셔틀 '그랩바디-B' 비임상 데이터 공개
- 빅파마, BBB 셔틀 기술 '쟁탈전'…뇌질환 정복 플랫폼 경쟁 가속
- ABL Bio’s Global Rise: Science, Strategy, and Speed
- ABL Bio Advances Global CNS Strategy with BBB Shuttle Platform

