Diversifying into Pharmaceuticals, Medical Devices, and Functional Foods to Strengthen Global Healthcare Presence
At an investor relations (IR) meeting held on January 3 at the Korea Exchange Conference Hall, Caregen CEO Yong-ji Jeong unveiled the company’s progress in developing therapies for wet age-related macular degeneration (wet AMD), pulmonary fibrosis, and dry eye syndrome. Alongside pharmaceutical innovations, Caregen emphasized its efforts to diversify into medical devices and health functional foods, positioning itself as a key player in the global healthcare market.

Wet AMD Treatment CG-T5: Interim Phase 1 Results
Wet AMD, characterized by abnormal blood vessel growth in the eye, is currently treated with injection therapies like Eylea. However, these treatments often impose a burden due to their repetitive and painful administration.
Caregen’s CG-T5 offers a novel alternative as an eye drop formulation that selectively binds to VEGFR-2, a critical protein in abnormal blood vessel formation. By blocking VEGFR-2, CG-T5 inhibits excessive vascular growth and inflammation, functioning as a “traffic cop” to restore order in the eye.
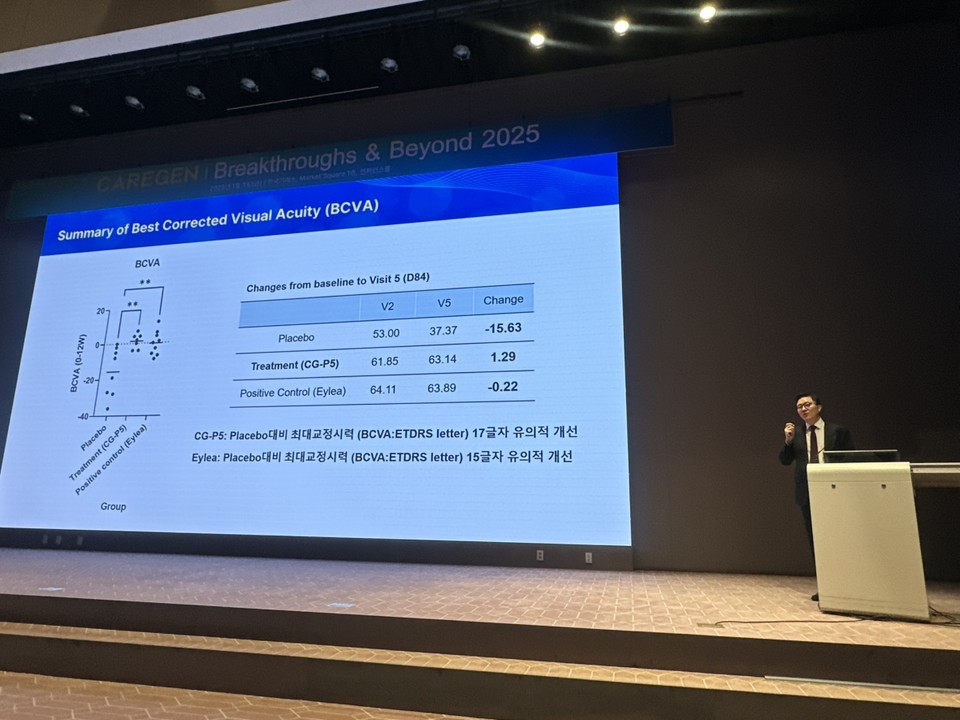
CEO Jeong presented interim Phase 1 results from U.S. clinical trials, demonstrating improvements in best-corrected visual acuity (BCVA) and central retinal thickness (CRT), along with strong safety and tolerability profiles. The company plans to expand CG-T5’s indications in Phase 2 trials to include diabetic retinopathy and other conditions.
COVID-19 to Pulmonary Fibrosis: The Journey of SpikeDown
Initially developed for COVID-19 treatment, SpikeDown has shown promise in addressing pulmonary fibrosis, a disease marked by progressive lung tissue hardening. Current treatments are limited, but SpikeDown functions as an ACE2 agonist, targeting the angiotensin pathway to reduce inflammation and fibrosis.
SpikeDown converts angiotensin II, which promotes inflammation and tissue damage, into angiotensin 1-7, an anti-inflammatory and tissue-repairing molecule. Binding to the MasR receptor, angiotensin 1-7 suppresses pro-inflammatory cytokines while boosting anti-inflammatory mediators, effectively inhibiting collagen accumulation and supporting tissue regeneration.
Animal studies have demonstrated SpikeDown’s potential to reduce inflammation and slow fibrosis progression, offering hope for this challenging condition.

Dry Eye Treatment T1: Boosting Tear Production and Reducing Inflammation
T1, Caregen’s candidate for dry eye syndrome, leverages a unique mechanism targeting the PAC1 receptor to stimulate mucin and other proteins critical for tear stability. This process enhances tear retention, reduces dryness, and alleviates inflammation.
In preclinical studies, T1 outperformed existing treatments, including cyclosporine-based therapies, by repairing damaged corneal tissue and maintaining tear film stability. Unlike cyclosporine, T1 was well-tolerated, minimizing irritation and improving patient comfort.
Caregen plans to complete preclinical development for T1 by year-end and submit an Investigational New Drug (IND) application to the U.S. FDA.