Chairman Lee: "Following Big Pharma Won't Ensure Global Leadership"
Calls for 'Bio Asia' and 'ACH' Regulatory Framework
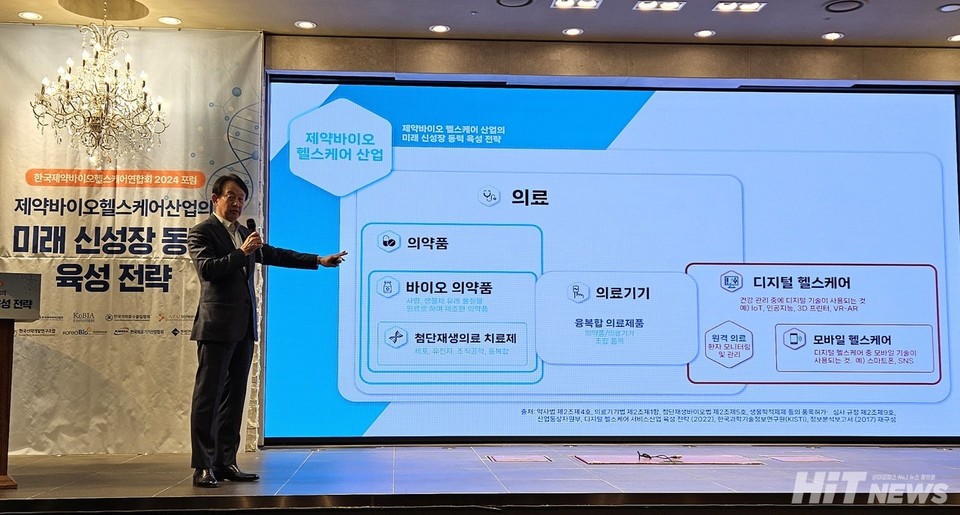
To achieve global recognition in the pharmaceutical and biotech ecosystem, largely dominated by U.S. and European regulations and multinational corporations, South Korean companies must explore an Asia-centered cooperative framework.
Byung-Geon Lee, Chairman of GI Innovation and member of the National Strategic Advanced Industry Committee, shared his perspective at the "Strategies for Fostering Future Growth Engines in the Pharmaceutical, Biotech, and Healthcare Industries" forum, organized by the Korea Pharmaceutical Bio-Healthcare Association on September 10th.
Moderated by Eui-Kyung Lee, Professor at Sungkyunkwan University College of Pharmacy and former Commissioner of the Korea Food and Drug Administration, the forum included discussions by prominent figures like Dae-Hee Shin, Vice President of LSK Global Pharma Services, Ki-Yong Hong, Professor of Business Administration at Incheon National University, and Yu-Kyung Hwang, Advisor to the Advanced Regenerative Medicine Industry Policy Committee, among others.
The Korea Pharmaceutical Bio-Healthcare Association represents eight organizations, including the Korea Pharmaceutical and Bio-Pharma Manufacturers Association, the Korea Biotechnology Industry Organization, and the Korea Digital Healthcare Industry Association.
Chairman Lee stressed that South Korean companies, already at a financial and regulatory disadvantage compared to global "Big Pharma," cannot rely on mimicking the blockbuster-driven models of larger firms to achieve global leadership.

Chairman Lee explained, “South Korea faces challenges in becoming a global leader with its current approach. For example, Eli Lilly's research budget alone is $12.7 billion, while the combined research budgets of all South Korean pharmaceutical and biotech companies total less than $3.7 billion. Even with $1.5 billion in government support, the gap remains significant.”
He further noted that many South Korean biotech firms are investing in substances to enhance the efficacy of existing blockbuster drugs, such as Keytruda, rather than developing innovative new drugs. This approach not only increases the risk of technology leakage but also drains substantial research funds.
Lee also questioned the necessity of Phase 3 clinical trials, dominated by global Big Pharma, for many treatments. He suggested that safety and efficacy could be established through Phase 1 and 2 trials in most cases, and proposed a regulatory reform modeled after Japan’s system, where drugs with proven safety are first used and then re-evaluated.

Lee called for an integrated Asian summit to shift the current paradigm, which is dominated by U.S. and European pharmaceutical, biotech, and healthcare industries.
He noted, "At global events like Bio USA or Bio Europe, Big Pharma companies hold hundreds of meetings, resulting in just one or two contracts. Asia, with its 4.5 billion population, must unite to form a 'Bio Asia,' a platform for cooperation at both governmental and public-private levels."
He also proposed creating the "ACH (Asian Council for Harmonization)," an Asian version of the globally harmonized ICH guidelines, to ensure regulatory autonomy from U.S. and European influence. Lee emphasized the importance of collaboration between the public and private sectors, with government participation to develop this framework further.

Lee outlined a two-track strategy for companies developing treatments in emerging sectors like neurological diseases and anti-aging therapies.
Initially, companies may need to license technologies to advanced markets like the U.S. and Europe. However, by retaining rights to the Korean and Asian markets, as seen in the case of Leclaza, they can secure future royalties. Conducting their own clinical trials and regulatory processes will also build valuable expertise.
He concluded by highlighting the potential of anti-aging therapies as a differentiated field, which, if linked with South Korea’s medical tourism industry, could become a core growth sector.
관련기사
- "K-제약바이오 글로벌 도약, 아시아 중심의 협의체 운영 필요"
- '산학연병정' 협력 생태계 구축, '현장 목소리' 기반한 정부 지원 절실
- 제약바이오서 쌓은 30년 내공 최수진 의원... "쓰임새 있는 정치하고 싶어"
- 융자 지원하고, 일부분 삭감안되는 'R&D 블루오션 개척' 법개정 추진
- 최수진 당선자 "바이오 M&A 활발…벤처 생태계 확장 모델 필요"
- 바이오협·의료기기협 등 3개 단체, 제약바이오헬스케어연합회 합류
- "규제 잘하면 국내 제약바이오기업 수출에도 도움"
- 국내 6개 단체 연합, '한국제약바이오헬스케어연합회' 출범
- 한국제약바이오협회, 지식재산전문위원회 출범

