Contractor Switch Delayed, Approval Expected Q3
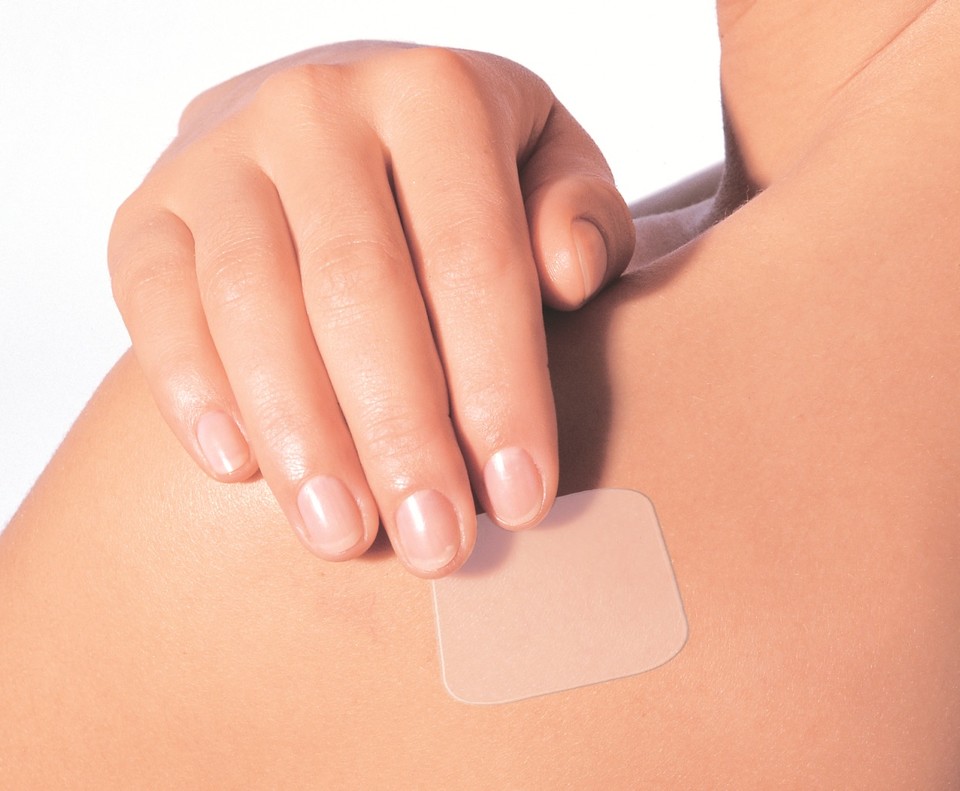
Hanmi Pharmaceutical has failed to meet its agreed production volume of Tooburol (tulobuterol patch), a pediatric cough medicine whose price was raised in 2024 to address supply instability. The company attributed the shortfall to delays in obtaining approval for a new contract manufacturer and expects the process to be finalized by the third quarter.
According to industry sources on August 19, the National Health Insurance Service (NHIS) is reviewing whether tulobuterol patch manufacturers have fulfilled their commitments to increase output following last year’s price adjustment.
To stabilize essential medicine supplies, the NHIS introduced new guidelines requiring manufacturers to submit cost statements, audit reports, and transaction records. In return for higher reimbursement prices, companies pledged to secure production equivalent to 13 months of demand.
As part of this agreement, 16 companies—including Sam-A, Arlico, Boryung Biopharma, Ilsung, Reyon, Yungjin, Union, Dae Hwa, Kolon, PharmGen Science, Chong Kun Dang, Alvogen Korea, Hanwha, JW, Hanmi, and GL Pharma—committed to boosting supply. The reimbursement ceiling for tulobuterol patches was raised in March 2024 by up to 25%, with prices increasing to $0.35–0.41 for the 2 mg patch, $0.23–0.25 for 1 mg, and $0.15–0.16 for 0.5 mg.
Thirteen months later, the NHIS began verifying compliance. Hanmi was found to have fallen short.
The issue stems from production capacity. While 17 companies market the tulobuterol patch, only Shinil and Dae Hwa manufacture it. Hanmi’s original contractor ceased supply, forcing a switch to Shinil. However, regulatory approval for this change was delayed by the Ministry of Food and Drug Safety.
Shinil has since fulfilled its obligations, and other companies outsourcing to Shinil also met their targets, alleviating broader supply concerns. Hanmi, however, remains behind schedule, raising questions over accountability as the price hike was conditional on supply commitments.

