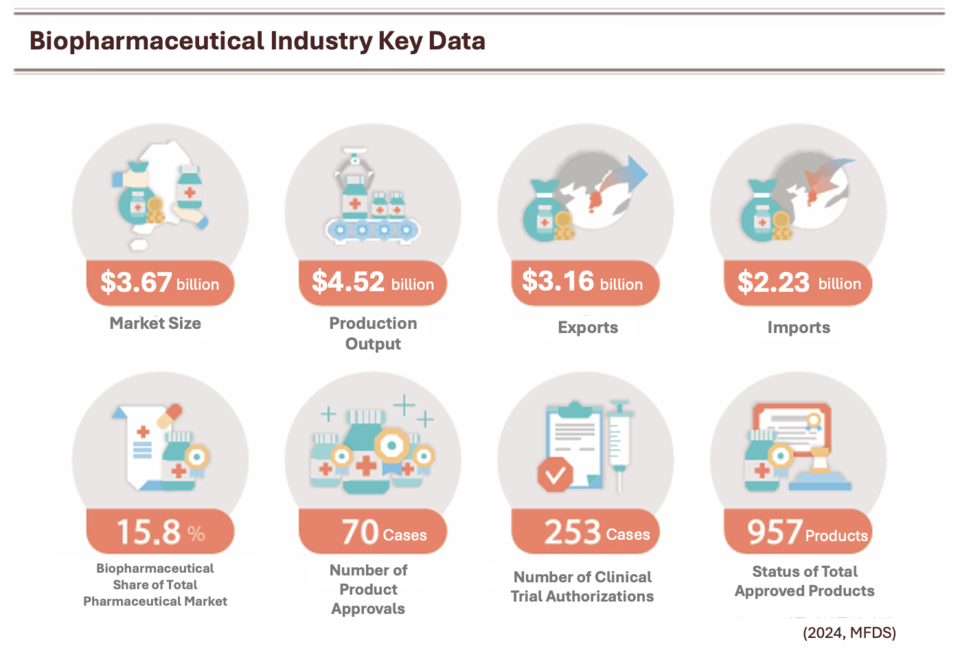
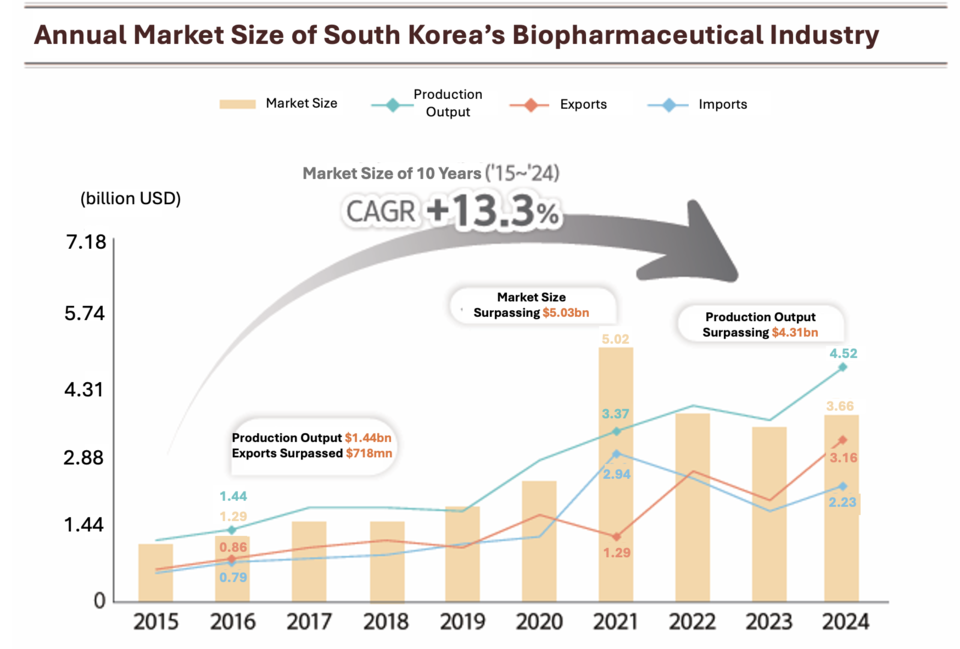
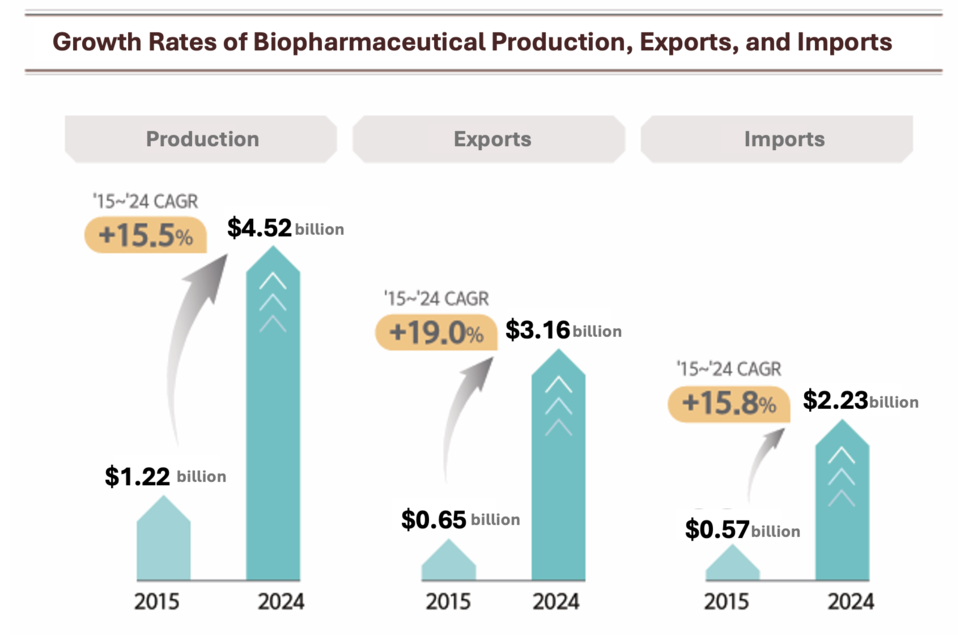
South Korea’s biopharmaceutical market reached approximately $3.59 billion in 2024, sustaining a compound annual growth rate (CAGR) of 13.3% from 2015 to 2024, according to the Korea Biopharmaceutical Association. Production output rose 26.4% year-on-year to $4.52 billion.
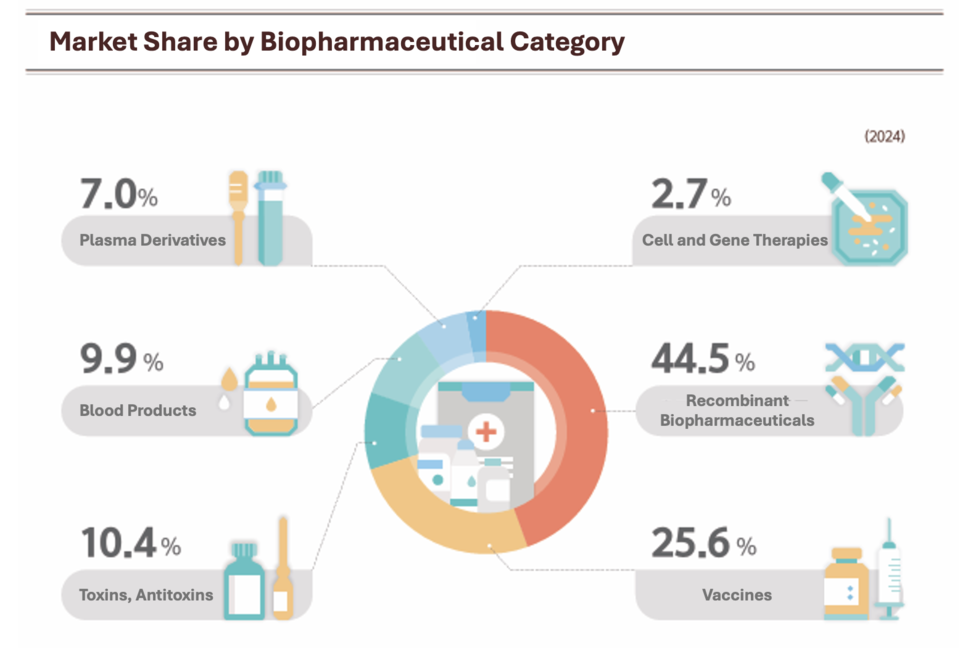
By product category, recombinant biopharmaceuticals led with a 44.5% market share, followed by vaccines (25.6%), toxins and antitoxins (10.4%), blood products (9.9%), plasma derivatives (7.0%), and cell and gene therapies (2.7%).
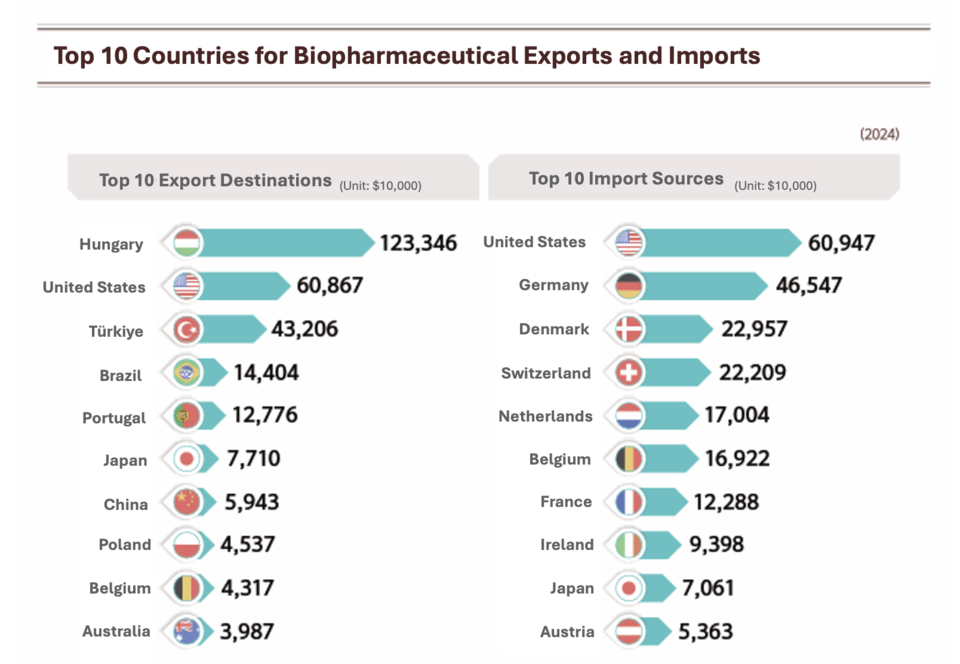
The trade surplus for biopharmaceuticals hit an all-time high of $916.92 million. Hungary was the top export destination at $1.23 billion, ahead of the United States and Türkiye. On the import side, the United States ranked first with $609.47 million, followed by Germany and Switzerland.
By the end of 2024, South Korea had approved a cumulative total of 957 biopharmaceuticals: 479 recombinant biopharmaceuticals, 192 blood products, 160 vaccines, 57 plasma derivatives, and 17 cell and gene therapies.
The Key Data on Korea’s Biopharmaceutical Industry 2025 is based on 2024 statistics released by the Ministry of Food and Drug Safety in 2025.

