Building a Full-Spectrum Drug Development Platform Beyond CDMO
Samsung Biologics is accelerating its entry into the clinical research organization (CRO) sector through the launch of a cancer-derived organoid platform. The move signals the company’s broader strategy to expand beyond its established role as a contract development and manufacturing organization (CDMO) and offer integrated, end-to-end drug development services.
At the BIO USA 2025 conference held on June 17 at the Boston Convention Center, Samsung Biologics unveiled its latest strategic initiative centered around organoid-based research.
“Cancer-derived organoids are a powerful tool for elucidating the mechanism of action (MOA) of new drug candidates,” said Sang-Myung Lee. “Rather than targeting immediate profits, this platform lays the groundwork for Samsung Biologics’ transformation from a CDMO into a CRO.”
This strategic shift aligns with recent regulatory developments. The U.S. Food and Drug Administration (FDA) has announced plans to gradually replace animal testing with organoid models and transition its drug review process to a generative AI-based system.
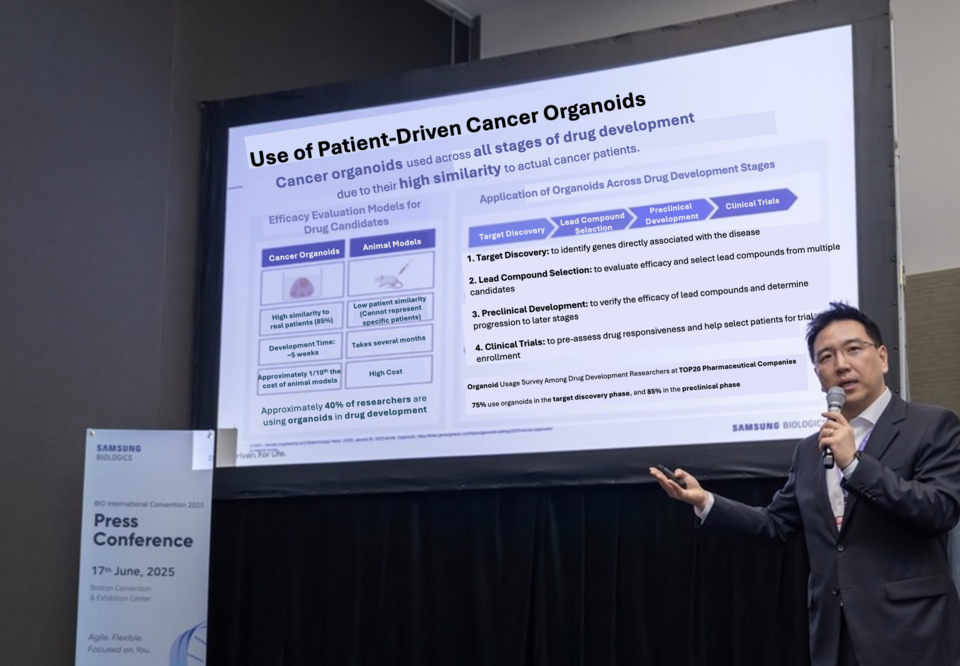
Lee emphasized that although the organoid market is still nascent, advances in AI and genomic technologies are expected to drive rapid growth. “With regulatory support and technological readiness converging, now is the opportune time to enter this space,” he said.
Since its entry into contract manufacturing (CMO) in 2011 and development (CDO) in 2018, Samsung Biologics has steadily expanded its service offerings. The organoid initiative marks the final step in completing its integrated biopharma platform, encompassing manufacturing, development, and now research.
A recent internal survey conducted by Samsung Biologics revealed that 75% of 70 drug development researchers from 20 pharmaceutical companies already use organoids during candidate discovery, and 85% in preclinical studies.
“While the organoid market remains small, interest in applying these models for anti-cancer drug discovery is rising,” said Lee. “If we can build a solid data foundation and validate performance, it may be possible to base licensing deals solely on organoid-derived data.”
Despite their biomimetic advantages, organoids face limitations such as variability and low scalability. However, Samsung Biologics is addressing these challenges through its partnership with Samsung Medical Center, securing diverse cancer organoid samples and implementing strict anonymization protocols to ensure high-quality, customized data for drug development.

