Featuring Huons Group’s Drug Products and Innovations from Huons, Huonslab, and Pangen Biotech
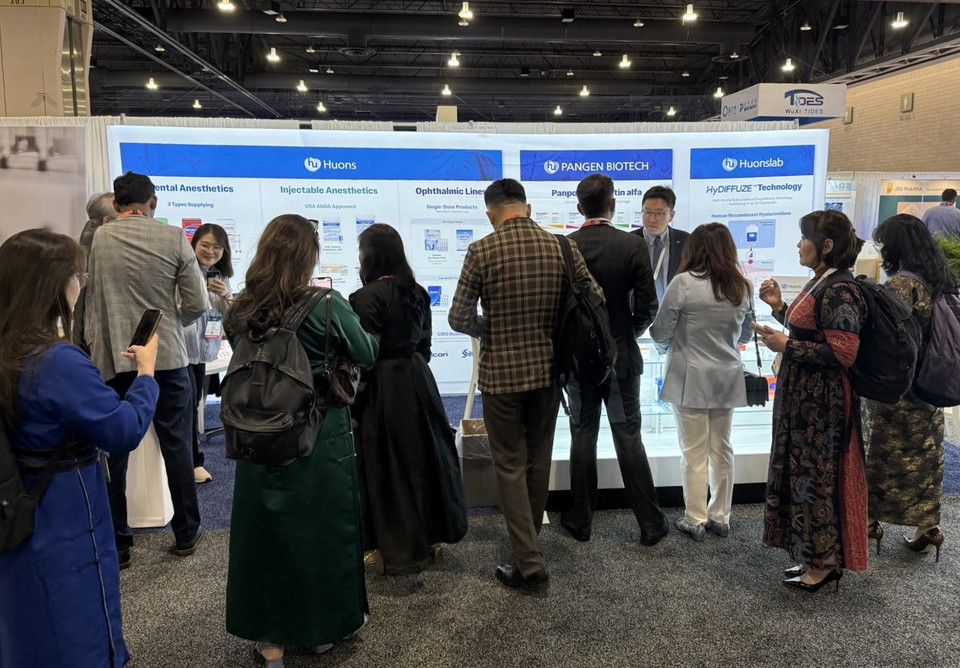
Huons USA, the U.S. sales arm of Huons Group, promoted the Group’s pharmaceutical products and innovative drug pipelines at the CPHI Americas exhibition held at the Pennsylvania Convention Center in Philadelphia.
The event, formerly known as CPHI North America, drew thousands of participants from over 500 pharmaceutical companies, distributors, and R&D institutions across more than 50 countries.
At the exhibition, Huons USA highlighted a comprehensive product portfolio, including key offerings from Huons, Huonslab, and Pangen Biotech. The spotlight was on Huons’ renowned injections and eye drops—particularly the newly FDA-approved 1% and 2% lidocaine hydrochloride multi-dose vials (2mL), which garnered significant attention.
Pangen Biotech’s Erythropoietin (EPO) biosimilar and CDMO (contract development and manufacturing organization) services for biopharmaceuticals were also introduced. Meanwhile, Huonslab presented its proprietary platform technology, HyDIFFUZE™, which utilizes recombinant human hyaluronidase to enable the conversion of intravenous (IV) infusions to subcutaneous (SC) injections.
Throughout the convention, Huons USA actively engaged in meetings and business discussions with buyers and distributors from Latin America, the Middle East, Asia, and Africa.
Huons USA CEO Choi Jae-myung commented, “At CPHI Americas, we focused on elevating Huons Group’s global visibility and strengthening brand competitiveness. We look forward to continuing discussions with partners to explore new collaborations and export opportunities.”

