Strategic Partnership with Roche Revives NSCLC Drug Amid Market Shifts

Starting in March, Boryung will take over the distribution and sales of Tarceva (erlotinib) in Korea, along with marketing authorization. Tarceva, an EGFR-targeted therapy for locally advanced or metastatic NSCLC, was once a market leader, generating over $13.9 million in sales before the rise of generics. Notably, Boryung, which contributed to Tarceva’s success, is resuming its role under the new rights-acquisition arrangement five years after their prior agreement ended.
Roche first launched Tarceva in Korea in 2006 after securing approval in 2005. However, the drug’s patent expiration in November 2016 led to fierce competition from generics introduced by local pharmaceutical firms such as Chong Kun Dang, Handok Teva, and HK inno.N. To maintain its market presence, Roche partnered with Boryung in 2017, leveraging Boryung’s well-established oncology sales team. Interestingly, despite securing approval for its own generic version, Boryung chose to promote the original drug instead.
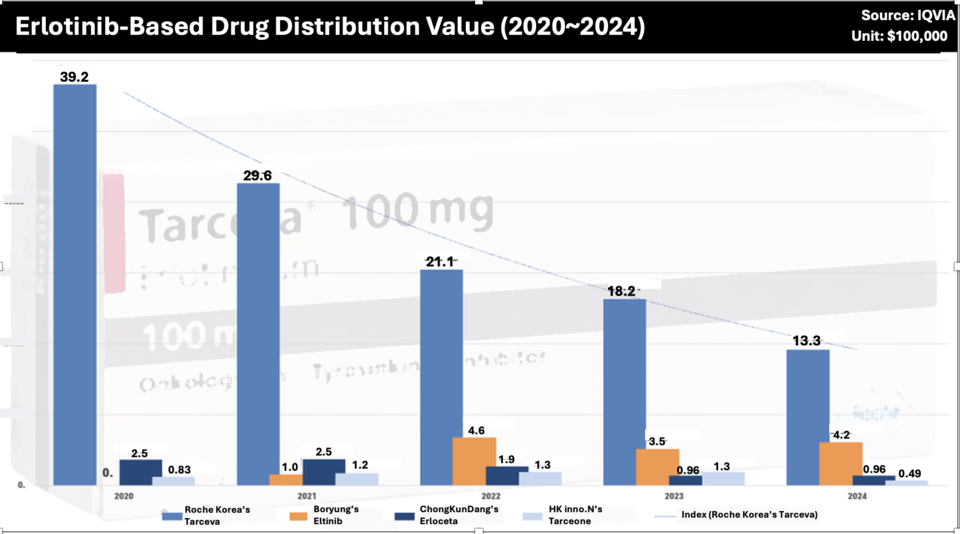
The co-promotion lasted about four years before Boryung and Roche parted ways in 2020. Following their split, Tarceva’s performance declined sharply. According to IQVIA data, its distribution value fell from $3.9 million in 2020 to $2.9 million in 2021, then further declined to $2.1 million in 2022, $1.8 million in 2023, and $1.3 million in 2024. Market analysts attribute this downturn to the emergence of next-generation lung cancer therapies, which have displaced older treatments like Tarceva. Competing drugs from Boryung, Chong Kun Dang, and HK inno.N have also reported annual sales below $700,000, reflecting the market’s shift toward newer therapies.
The renewed partnership between Boryung and Roche indicates strategic benefits for both sides. Roche is prioritizing newer oncology treatments like Tecentriq, shifting focus to its next-generation pipeline. Meanwhile, Boryung continues strengthening its oncology portfolio with key chemotherapy agents such as Gemzar and aims to position itself in the growing generics market.
Although Tarceva’s current market share is limited, it was once a dominant force in NSCLC treatment. Boryung may view this as an opportunity to leverage Tarceva’s legacy and integrate it into broader oncology strategies. The positive track record from their previous collaboration likely played a role in Roche’s decision to entrust Boryung with the product once again.
Article Correction Notice
Roche Korea clarified that, under a global transfer agreement, Roche headquarters transferred the Tarceva rights to Chepla Pharm. Chepla Pharm has designated Boryung as the South Korean license holder, with sales set to begin in March.
Accordingly, the reference to co-promotion has been updated to indicate that Boryung’s sales, commencing on March 1, will follow the rights acquisition.

