Law for AI industry Growth and Regulation

The “Framework Act on Artificial Intelligence” (hereafter referred to as the AI Framework Act) has passed the National Assembly, while AI-powered medical devices are expected to follow the Digital Medical Products Act, set to take effect in January 2025.
According to the National Assembly, the AI Framework Act was approved in a plenary session on December 26. Initially proposed in July 2020 and developed over four years through extensive public input, the Act aims to establish a foundation for the trustworthy use of AI technologies.
The Ministry of Science and ICT, which oversees the AI Framework Act, stated that the law defines high-impact AI and generative AI as regulatory targets to prevent potential issues stemming from technical limitations, misuse, or abuse. It mandates obligations for transparency and safety while specifying the responsibilities of AI businesses. Additionally, it provides a legal basis for government support of voluntary AI safety and reliability certification systems and AI impact assessments initiated by the private sector. The Ministry emphasized the importance of clear regulations and building a foundation for safety and trust.
The AI Framework Act will undergo a one-year preparatory period and is scheduled to take effect in January 2026. However, in the field of medical devices, the Digital Medical Products Act will come into force immediately on January 24.
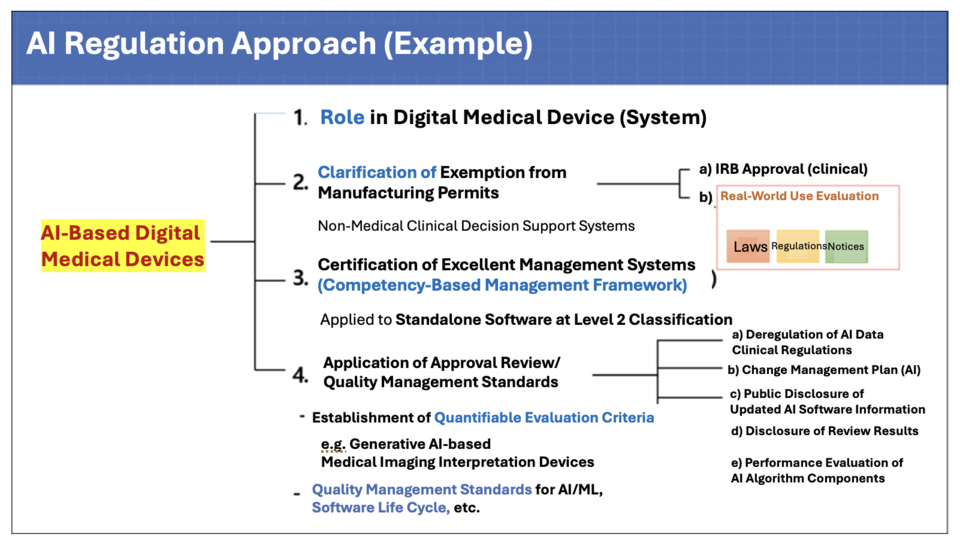
During a recent briefing on the Digital Medical Products Act, Byung-kwan Kim, an official from the Digital Medical Products Task Force of the Ministry of Food and Drug Safety (MFDS), clarified, “AI regulations are also addressed under the AI Framework Act, but AI-powered medical devices will follow the Digital Medical Products Act and will not be subject to additional regulations. This avoids regulatory overlap by incorporating relevant provisions of the AI Framework Act into the Digital Medical Products Act.”
The Digital Medical Products Act introduces a distinct management system for AI-powered medical devices, including assigning unique classification codes. Given their nature of frequent updates, modifications falling within predefined boundaries can be managed through a pre-submitted update management plan instead of requiring change approval, differing from conventional medical devices.
Provisions outlined in the AI Framework Act, such as consumer notification obligations regarding the application of AI technologies and the establishment of risk management systems, are also incorporated into the Digital Medical Products Act.
The AI Framework Act defines "high-impact AI" systems as those that may significantly affect or pose risks to human life, physical safety, and fundamental rights. This category includes AI systems used in medical devices, energy management, drinking water, healthcare, nuclear facility operations, biometric information for criminal investigations, decisions affecting individual rights and obligations such as hiring and loan evaluations, transportation, student assessments in early and secondary education, and other areas specified by presidential decree.
Beyond regulation, the AI Framework Act also aims to foster industry development. A representative from a domestic AI medical device company commented, “The Act includes initiatives to support AI-related projects such as building AI training datasets, promoting AI adoption and application, supporting technology development, providing special assistance for small and venture enterprises, aiding startups, and facilitating entry into international markets. These measures are expected to contribute to the advancement of AI-powered medical devices.”

