Attorney Johana Cho Challenges Drug Approval Delays: 'Why Are Life-Saving Medications Not Reaching Patients Faster?'
A Patient Asks YoonCare – Neuroblastoma
Attorney Johana Cho of the law firm Bae, Kim & Lee LLC, faced an overwhelming ordeal when her child was diagnosed with stage 4 neuroblastoma at just under three years old. Despite the emotional weight of the diagnosis, Cho remained strong for her child, adopting a positive approach to make hospital visits less daunting.
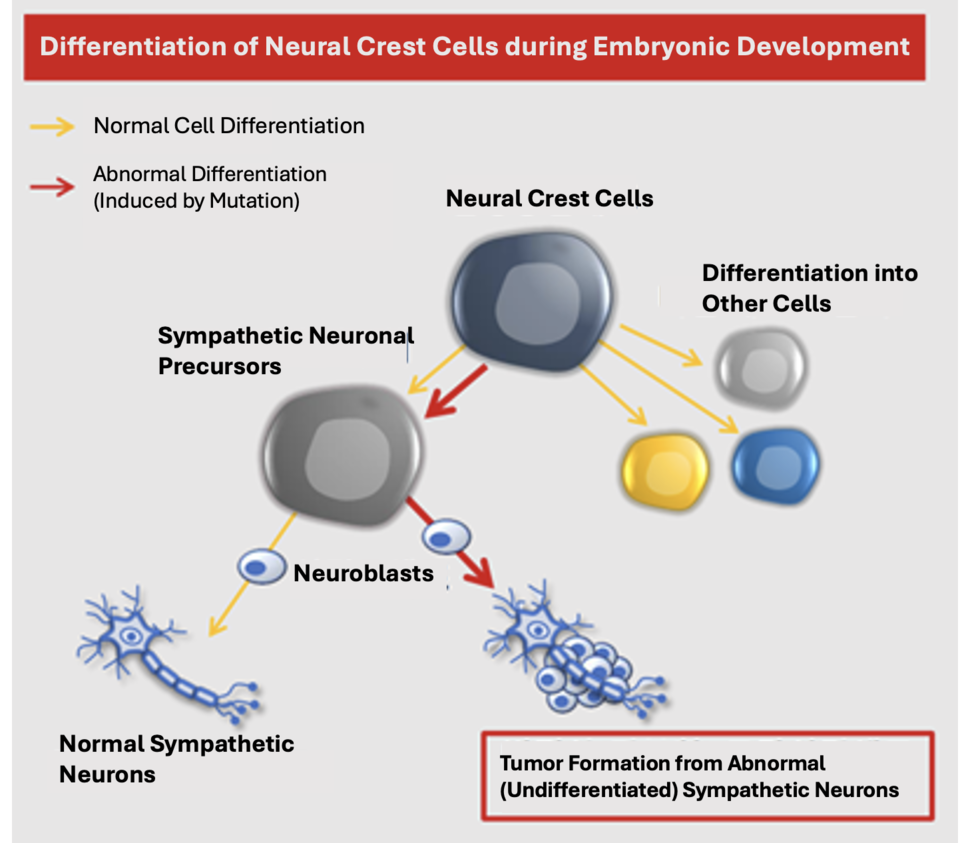
Neuroblastoma is a rare childhood tumor originating from neuroblasts that fail to mature. Diagnosed mostly in children under five, this cancer accounts for 15% of childhood cancer deaths. Despite advances, the prognosis remains challenging, with high-risk neuroblastoma yielding a long-term survival rate of 40-50%, dropping to 20% for relapsed cases.

Attorney Cho shared, “My child’s persistent abdominal pain was first misdiagnosed as enteritis twice. I felt uneasy when symptoms persisted despite treatment. After a CT scan, the diagnosis shifted to high-risk neuroblastoma.” The diagnosis began Cho's quest for information, scouring research and joining online forums to understand available treatments. Yet, the most challenging aspect was managing her child’s limited daily activities, especially during a month-long fast required for chemotherapy. Cho recalled her child asking, “Can’t I eat something?” – a question that broke her heart. Despite the hardships, she strove to make the hospital experience as positive as possible, creating moments of joy and comfort.
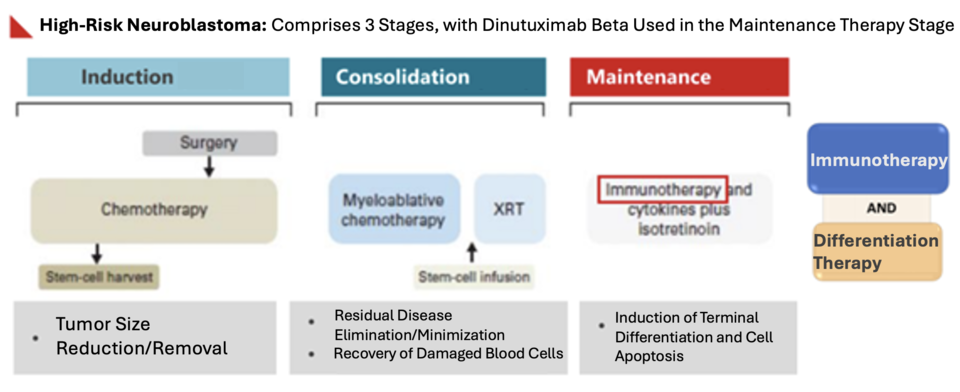
The standard high-risk neuroblastoma treatment includes induction, consolidation, and maintenance stages across nine chemotherapy rounds. Surgery, high-dose chemotherapy, autologous stem cell transplants, and targeted radiation using mlBG are involved. Cho’s child, however, grew weak after the first transplant, making a second one too risky. Instead, the child completed mlBG and proton therapy and now takes Isotretinoin to suppress malignancies.
In another case, a nine-year-old boy, relapsed three months after initially successful treatment. Now hospitalized for three months, he began treatment with Qarziba (dinutuximab beta), a drug approved by the Ministry of Food and Drug Safety (MFDS) in June for high-risk and relapsed neuroblastoma.
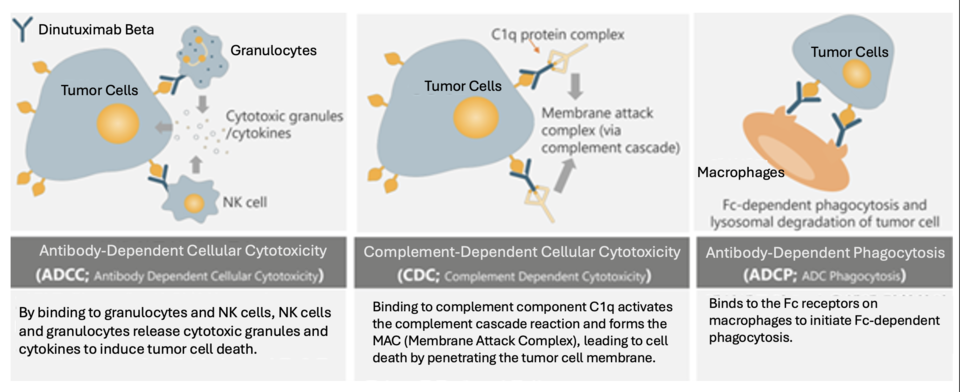
Qarziba, distributed by Recordati Korea, is an immunotherapy that targets GD2, a protein on neuroblastoma cells, to stimulate tumor cell death. Currently, it is under review for insurance coverage. Patient A’s guardian expressed frustration, noting, “Other countries offer new drugs more quickly. Why must we wait in Korea?” With the financial burden of uninsured drugs, they stressed the impact on families, especially those with young children and limited economic participation.
Attorney Cho highlighted a key limitation: current coverage only applies to patients who recently completed high-dose chemotherapy and transplantation. This excludes children too weak for a second transplant, despite their need for alternative treatments. “I hope Qarziba’s coverage extends soon for these children. Without a second stem cell transplant, survival rates drop, underscoring the need for accessible treatments.”
Cho also called for more input from patients and families on drug coverage policies. “It’s disappointing that parents lack a voice on what treatments are covered. Allowing feedback could guide insurance decisions and better align with patient needs.”
Additionally, Cho proposed a government-backed insurance system for newborns to alleviate treatment costs. “Child insurance for rare diseases could be structured to ease burdens on affected families. This investment would minimally impact societal costs but greatly support families.”


