Over 10 Companies Already Supplying Single and Combination Products
Non-Green List Status Avoices Patent Infringement, Frustration with Multinational Firms
Despite receiving a certified mail warning from Boehringer Ingelheim, South Korean pharmaceutical companies have proceeded with releasing generic versions of the diabetes drug Trajenta. This defiant move is based on the belief that items not listed on the MFDS “green list” incur minimal damages even if litigation occurs, and that their actions do not violate the law.
As of June 10, around ten companies, including Daewoong Bio, Withus Pharmaceutical, Kyongbo Pharmaceutical, and Arlico Pharmaceutical, led by Myungmoon Pharmaceutical, have reported selling generic versions of Trajenta. These companies released single and combination products following the expiration of the substance patent for the original linagliptin formulation 'Trajenta Duo' on June 8.
In late May, Boehringer Ingelheim's global headquarters, through a law firm, issued a notice stating that generic developers had infringed on the patent and warned of seeking injunctions and damages if generics were launched. They emphasized that, despite 127 products from 19 companies being granted priority sales approval for single agents as of June 9, six patents not listed on the MFDS green list could legally obstruct product sales.
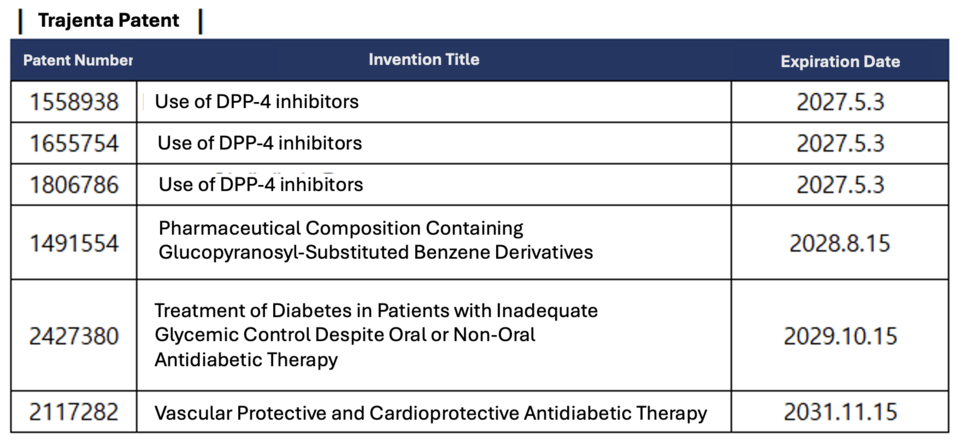
Additionally, Boehringer requested the generic manufacturers to disclose their intentions regarding the sale of products after June 9, 2024, their application for drug pricing, production status, and their basis for believing they do not infringe on Boehringer's patents.
Experts say that Korean pharmaceutical companies launched their products because the patents listed on the MFDS green list had expired. Under current regulations, patents for substances, compositions, formulations, and medical uses must be registered. However, during the evergreening strategy process, new patents were not registered after initial listing, allowing the launch of generics without legal issues.
A. According to Article 30-2, Paragraph 3 of the Enforcement Regulations of the Pharmaceutical Affairs Act, the patents related to drugs for which a registration application has been received must pertain to substances, compositions, formulations, or medical uses.
B. Drugs eligible for registration under item A include new drugs, new formulations, new compositions (items formulated with new active ingredients or excipients), and drugs with new efficacy, effects, or dosages (medical uses) as defined in Article 2, Paragraph 8 of the Pharmaceutical Affairs Act.
Even if a lawsuit arises, Korean pharmaceutical companies are unlikely to suffer significant losses. For instance, in a past legal battle over the generic version of 'Lyrica,' Pfizer won but received only around $10,000 in compensation due to low prescription incidence. As a result, potential lawsuit costs are minimal, reducing the apprehension of Korean companies.
The stringent patent strategies by multinational companies have caused frustration among Korean pharmaceutical companies. They have previously refrained from launching approved products due to issues with unregistered patents and have been filing for patent invalidation and avoidance trials. However, original manufacturers have raised these patents and sent certified mails, leading to criticism of unfair business practices.
Although the situation differs, AstraZeneca's certified mail to companies promoting an unlisted indication of Farxiga, which was later withdrawn, has influenced the current scenario. As Korean companies continue to launch generic drugs, attention is now focused on how both sides will react moving forward.

