Restructuring Aims to Strengthen Coordination and Efficiency in Approval Processes
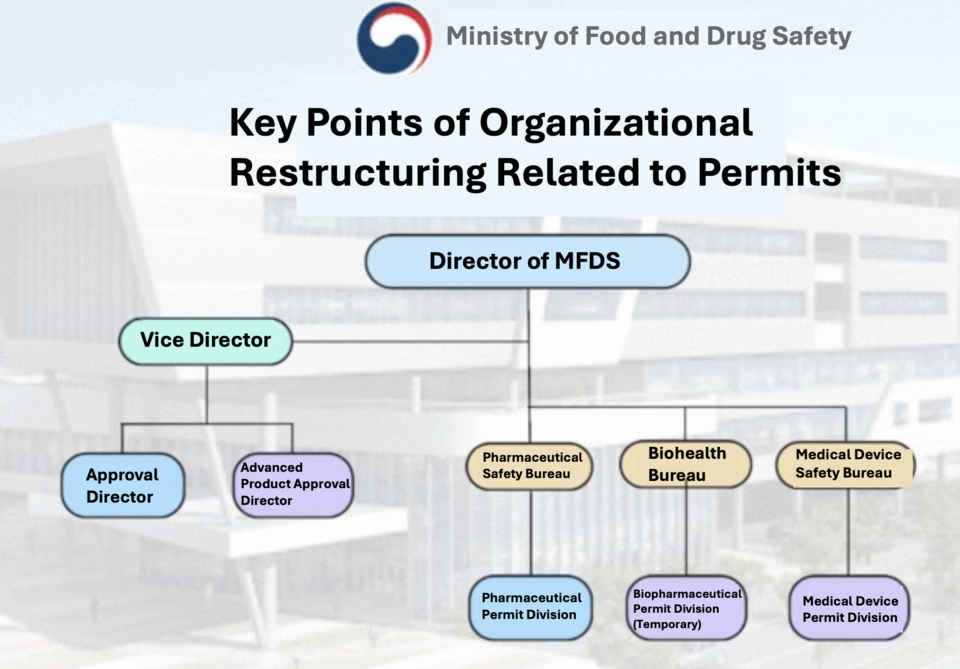
The Ministry of Food and Drug Safety (MFDS), led by Director Yoo-Kyung Oh, will implement a reorganization of the medical product approval department and its functions, commencing on May 7th. The objective is to bolster coordination between medical product approval and policy formulation.
According to the MFDS, effective May 7th, the roles of the Approval Director-General and the Advanced Product Approval Director-General, operating under the existing deputy director-general structure, will be eliminated. Instead, a new framework will be established within the Pharmaceutical Safety Bureau for overall pharmaceutical approvals, the Medical Device Safety Bureau for medical device approvals, and the Biohealth Bureau for bio-pharmaceutical policy, including the temporary establishment of the Bio Approval Team.
As part of this organizational overhaul, changes in jurisdiction over approved products will occur. Pharmaceuticals and hybrid medical products will come under the Pharmaceutical Safety Bureau's purview, while bio-pharmaceuticals (encompassing herbal medicine preparations and non-pharmaceutical products) will fall within the Biohealth Bureau's domain. Medical devices will be managed by the Medical Device Safety Bureau for approval tasks.
This restructuring initiative aims to comprehensively handle tasks such as product-specific manufacturing and import item approvals, policy formulation and application, and safety management within each policy and business bureau. Its goal is to enhance coordination between approval and policy functions while elevating expertise. Additionally, by integrating AI and other emerging technologies, the MFDS anticipates establishing an efficient approval system for innovative and advanced medical products.
Simultaneously, ongoing reorganization within the approval department seeks to modernize current functions with stakeholder needs in mind. This includes consolidating the innovation product consultation service into pre-consultation services and establishing a new approval and review coordination committee to streamline approvals and reviews. Furthermore, a system of continuous improvement in approval and review policies through regular quality assessments will be instituted, fostering a cycle of enhancement within the approval and review process.
Developers of innovative medical products, such as new drugs, will be able to swiftly designate a consultation department through pre-consultation services, facilitating systematic management of consultation records to enhance coordination between consultation and approval/review processes.
The approval-review coordination committee will pilot an official channel where applicants can directly request adjustments for supplements and other issues during the approval-review process. Under the supervision of the Director of the Pharmaceutical Safety Bureau, with internal and external experts participating as members, neutral review and adjustments of requested coordination issues will be facilitated. The MFDS plans to extend this initiative to bio-products and medical devices following an evaluation of the pilot operation's results.
Lastly, the MFDS intends to conduct regular quality assessments of the approval and review processes and share the findings with policy departments. This will establish a cyclical system for continuous improvement of approval and review policies, enabling proactive innovation in regulations and flexible adaptation to changes in the policy environment.
Officials from the MFDS expressed expectations for improvements in the accessibility and predictability of pre-consultation, along with enhanced reliability, predictability, and acceptability of approval and review outcomes. They anticipate increased efficiency in approval and review processes to support product commercialization. Furthermore, they reaffirmed their commitment to swiftly approving safe and effective medical products to ensure public safety and promote innovative policies that support the industry, enabling domestically developed medical products to lead the global market.
As part of this restructuring effort, certain functions of the medical product complaints handling system, including the electronic complaints portal, will be temporarily suspended for system maintenance related to the transfer of medical product approval application complaint handling departments on May 6th. For detailed information, individuals can refer to the notices and alerts within each complaints handling system.

