Approval Based on FLAURA2 Study Data Presented at IASLC 2023 WCLC Conference
AstraZeneca's targeted cancer therapy, Tagrisso (containing Osimertinib), combined with platinum-based chemotherapy, has received approval in Korea as a first-line treatment for EGFR mutation non-squamous non-small cell lung cancer, effective April 15th. This approval stems from AZ's FLAURA2 study data, which was unveiled at last year's International Association for the Study of Lung Cancer (IASLC 2023 WCLC) conference.
In February 2024, the US FDA sanctioned the concurrent usage of Osimertinib with chemotherapy, following the outcomes of the FLAURA2 study. This decision was echoed in the National Comprehensive Cancer Network (NCCN) guidelines, classified under "other recommendation." The study also facilitated additional indications for Tagrisso, which has achieved global annual sales of $5.1 billion.
The FLAURA2 clinical trial, a global phase 3, open-label study, targeted patients with advanced non-small cell lung cancer (NSCLC) harboring EGFR mutations (exon 19 deletion or L858R mutation) who had not undergone prior treatment for advanced disease. The study, comprising 557 patients, randomly assigned in a 1:1 ratio, investigated the efficacy of Osimertinib plus chemotherapy versus Osimertinib monotherapy.
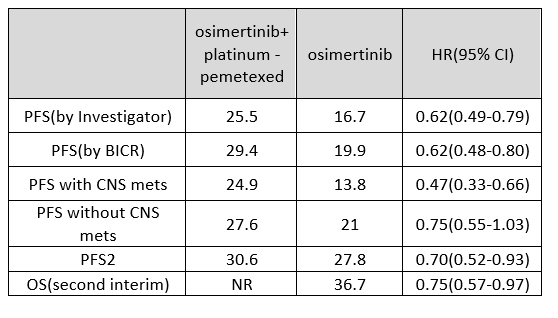
The primary endpoint of the FLAURA2 study was progression-free survival (PFS) based on investigator assessment, with results of 25.5 months versus 16.7 months (HR 0.62). Independently assessed (BICR) results indicated 29.4 months versus 19.9 months (HR 0.62), showcasing unusually high BICR evaluations, a rare occurrence raising concerns regarding study reliability.
The core of the study revolves around comparing the effects of upfront chemotherapy versus delayed therapy. In such scenarios, comparing the PFS of the combination therapy group with the PFS2 of the monotherapy group would be appropriate. However, the numerical difference between the PFS of 25.5 months in the combination therapy group and the PFS2 of 27.8 months in the monotherapy group challenges the assertion of combination therapy superiority in therapeutic effectiveness.
The FLAURA2 study underscores the significant advantages of combination therapy, particularly in patients with brain metastases. However, doubts arise regarding the validity of comparing PFS of 24.9 months versus 13.8 months in patients with brain metastases. Additionally, the discrepancy in the 13.8-month PFS in patients with CNS metastases treated with Osimertinib as monotherapy compared to the clinical results presented in FLAURA raises questions, especially regarding the purported differences between 3rd generation TKIs and 1st or 2nd generation TKIs.
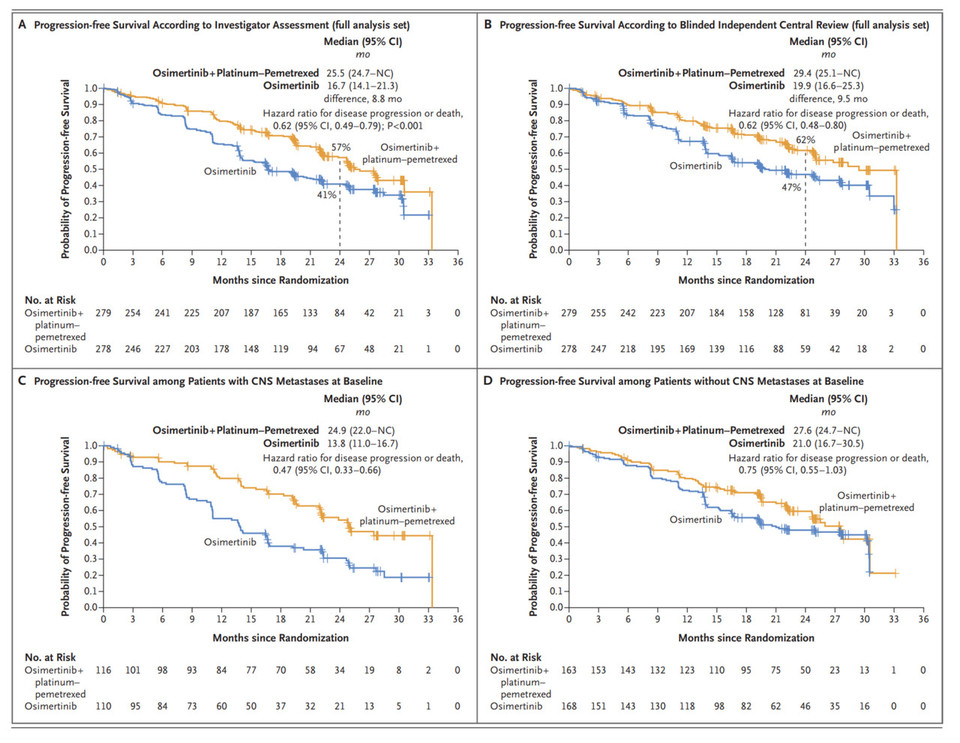
Moreover, highlighting the advantages of combination therapy in the absence of clear mechanisms supporting the impact of chemotherapy on brain metastases appears questionable. Toxicity issues associated with chemotherapy combination are noteworthy, with a higher rate of drug discontinuation due to adverse events observed in patients receiving platinum-pemetrexed therapy.
The ultimate determinant of treatment success lies in overall survival (OS). The OS update from FLAURA2, presented at the European Lung Cancer Congress in March 2024, with 41% maturity, revealed a median OS of NR (not reached) in the combination therapy group compared to 36.7 months in the monotherapy group, with an HR of 0.75. However, it did not achieve statistical significance with a p-value of 0.000001, indicating that subsequent treatment timing does not significantly impact patient survival.

