Global recognition for GLP-1 obesity treatments spurs development efforts by Peptron, ProGen, and More.
In the international landscape, the spotlight is on GLP-1 (Glucagon-like peptide-1) class treatments for obesity like Semaglutide and Tirzepatide, signaling a significant trend. Concurrently, numerous South Korean biotech entities are accelerating their endeavors in the domain of obesity treatments.
As per insights from the Mirae Asset Securities Research Center, the global market for GLP-1 class obesity treatments is projected to burgeon annually by 30%, reaching an impressive scale of $77.4 billion by 2030. This momentum has spurred South Korean biotech firms to actively engage in the development of obesity treatments, both independently and through collaborative ventures.
A roster of companies, including Peptron, ProGen, GI Biome, Olix Pharma, Inventage Lab, and Glaceum, are deeply involved in advancing obesity treatment development. Peptron, leveraging its 'SmartDepot' technology for sustained-release microsphere formulation, boasts GMP-certified production facilities and an in-house research lab. Notably, their pipeline includes 'PT404,' presently in preclinical stages for diabetes and obesity treatment, alongside a material transfer agreement (MTA) underway with a global pharmaceutical company.
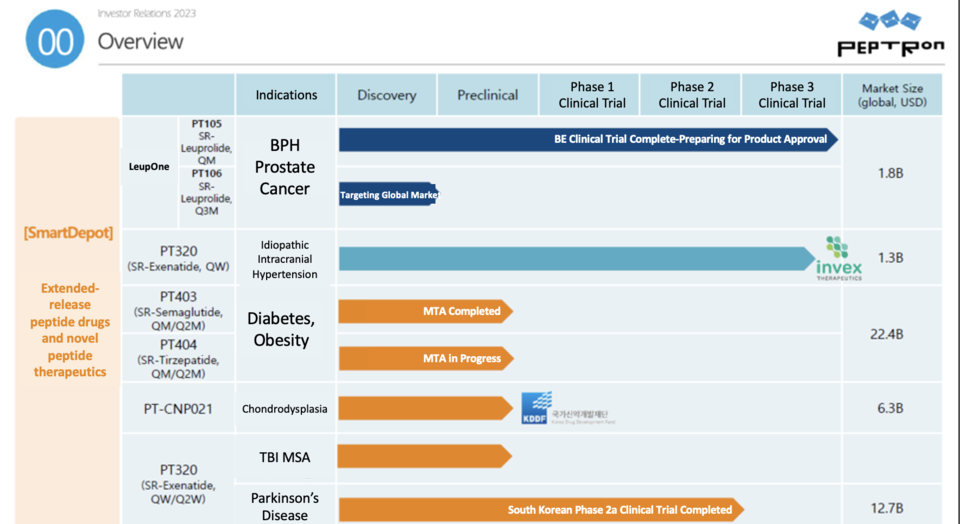
According to Kim Seungmin, a researcher at Mirae Asset Securities, Peptron is actively working on a 4-week formulation of 'WEGOVY (containing Semaglutide)' and 'Zepbound (containing Tirzepatide),' integrating Western technologies. Discussions for partnerships regarding the enhancement of these products from a 1-week to a 4-week format are currently in progress.
Previously, ProGen and GI Biome announced the signing of a Memorandum of Understanding (MOU) in July, aimed at jointly developing next-generation obesity treatments. This collaboration focuses on the combined research efforts of ProGen’s obesity treatment candidates and GI Biome’s microbiome targeting obesity and metabolic diseases, intending to maximize weight reduction effects, particularly in abdominal and visceral fat.
Olix Pharma recently unveiled the conclusive results of preclinical efficacy trials for their obesity treatment, 'OLX702A (development code name),' conducted on non-human primates in October. The trials showcased significant effects, including mitigated rebound weight gain, reduced body fat percentage, and decreased abdominal circumference, particularly in the group receiving OLX702A in combination with Semaglutide (marketed as Wegovy).
During the World Obesity Congress 2023 (WOC 2023), Inventage Lab disclosed the outcomes of non-clinical trials for their one-month long-acting injectable Semaglutide, 'IVL3021,' currently in developmental stages for diabetes and obesity treatments. Their focus lies in refining a 1-month long-acting prescription utilizing their proprietary 'IVL-DrugFluidic' technology.
Glaceum recently announced the signing of a material transfer agreement (MTA) with a global pharmaceutical company for their oral obesity treatment candidate compound, 'HSG4112.' This non-narcotic substance aims not only to treat obesity but also to prevent and manage cardiovascular diseases like non-alcoholic steatohepatitis (NASH), hyperlipidemia, and hypertension. Presently, the compound is undergoing phase 2 clinical trials.
Industry experts stress the significance of South Korean biotech firms in strategizing and setting directions to compete in the obesity treatment domain. A representative noted, "It's crucial to consider technological advancements for enhancing the sustained action of GLP-1 receptor agonists (GLP-1 RA) or providing additional therapeutic options beyond obesity for cardiovascular benefits to patients."
Moreover, they emphasized the importance of contemplating differentiated mechanisms of action for obesity treatment, targeting patients unresponsive to GLP-1 RA therapies, or considering combination therapies. Considering the vastness of the obesity treatment market, they recommended focusing on segmentation strategies to establish competitiveness instead of targeting the entire market.

