Presentations at ESMO 2023 highlight efficacy of Leclaza in Central Nervous System and Pemetrexed in Leptomeningeal metastasis

At the European Society for Medical Oncology (ESMO) 2023 Annual Congress in Madrid, Spain, held on October 22nd, significant findings were presented. These findings confirmed the effectiveness of "Leclaza" (active ingredient: Lazertinib) in central nervous system (CNS) and introduced the safety and efficacy of "Pemetrexed" (brand name: Pemedes) in patients with brain meningeal metastasis.
Leclaza, developed by Yuhan Pharmaceuticals, is a approved anticancer agent for the first-line treatment of EGFR-positive non-small cell lung cancer (NSCLC) patients and as a second-line treatment for EGFR T790M-positive patients. Brain metastasis is known to occur more frequently in EGFR-positive NSCLC patients, with an incidence approximately 1.8 times higher than other types. Moreover, the occurrence of brain metastasis tends to increase progressively following the diagnosis of lung cancer.
In the 'LASER301' Phase 3 clinical trial, which included 393 patients, 86 individuals with brain metastatic tumors were selected and divided into two subgroups: the Leclaza treatment group, consisting of 45 patients (25%), and the Gefitinib treatment group, comprising 41 patients (21%).
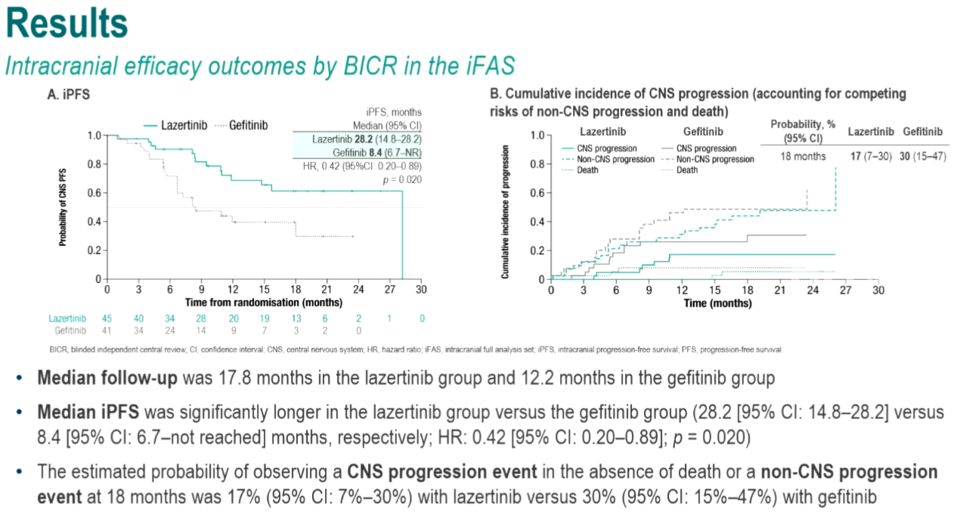
Presenting the study, Professor Ross Su from the National Cancer Centre Singapore disclosed that the median intracranial progression-free survival (iPFS) for the Leclaza treatment group was 28.2 months (95% CI: 14.8-28.2), while for the Gefitinib group, it was 8.4 months (95% CI: 6.7-NR). Leclaza demonstrated a 58% reduction in the risk of disease progression or death (HR=0.42, 95% CI: 0.20-0.89, P=0.020).
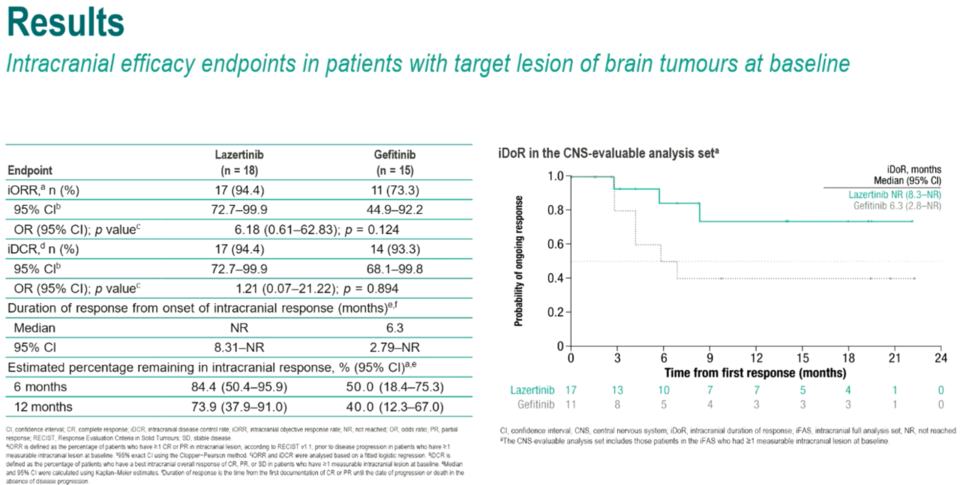
Intracranial response rates (iORR) stood at 94.4% for the Leclaza group and 73.3% for the Gefitinib group. The median duration of intracranial response (iDoR) was not reached for the Leclaza group (95% CI: 8.3-NR), while it was 6.3 months (95% CI: 2.8-NR) for the Gefitinib group.
In assessing the efficacy of central nervous system disease progression control, the rates of disease progression within the central nervous system at 6 months and 12 months were 5% and 17% for the Leclaza group, respectively, and 18% and 26% for the Gefitinib group, respectively.

Department of Hematology and Oncology
Commenting on the study, Professor Beom-Seok Kim from the Department of Hematology and Oncology at Seoul National University Hospital stated, "Prior to these results, we had suspected Leclaza's potential for brain metastasis based on medical imaging data. However, the significance here lies in the emergence of data that confirm this hypothesis for the first time."
He continued, "As brain metastasis progresses, patients may experience symptoms such as headaches and paralysis, and in severe cases, one side of the body may become paralyzed. Since the overall prognosis for such patients is unfavorable, many succumb without the benefit of chemotherapy or radiation therapy." Professor Kim added, "In this regard, Leclaza, as a third-generation EGFR mutation-targeted anticancer agent, plays a vital role in reducing brain metastasis, a development of great significance to clinicians. Ultimately, it also implies that people without brain metastasis may be able to prevent its occurrence."
In essence, Professor Kim's perspective is that Leclaza, with its superior penetration of the blood-brain barrier (BBB) compared to some cytotoxic anticancer agents, not only aids in treating brain metastasis but also in preventing the progression of central nervous system diseases.

Meanwhile, this year's ESMO featured the results of the 'LAZARUS' study, led by researchers, which explored the efficacy and safety of Leclaza and Pemetrexed combination therapy in EGFR mutation-positive lung cancer patients with leptomeningeal metastasis (LMS). Leptomeninges are the thin membranes surrounding the brain, and when leptomeningeal metastasis occurs, symptoms can manifest throughout the body.
According to the researchers, patients with leptomeningeal metastasis have a median survival of just 4 to 6 weeks if left untreated. While there is a treatment method that involves intrathecal administration of chemotherapy drugs, even this approach results in a median survival of only 2 to 3 months.
The researchers explained, "While EGFR TKI targeted therapy can be attempted for EGFR mutation-positive patients, the limited penetration of 1st and 2nd generation drugs through the blood-brain barrier (BBB) may restrict their therapeutic efficacy."
In response, the research team initiated a combination therapy involving Leclaza and Pemetrexed for leptomeningeal metastasis in EGFR mutation-positive lung cancer patients. Subsequently, they conducted periodic cerebrospinal fluid examinations to determine the concentration of Leclaza in the cerebrospinal fluid.
Three weeks after initiating treatment, the average Leclaza concentration ratio in cerebrospinal fluid (CSF) compared to free plasma was 77%, with a median value of 50%. This indicates a higher concentration in the central nervous system (CNS) compared to traditional EGFR TKIs. While the follow-up period remains insufficient to determine treatment efficacy, such as extended survival, Professor Beom-Seok Kim emphasized, "When metastasis is detected through cerebrospinal fluid analysis, treatment options are limited, and the patient's condition deteriorates rapidly."
He added, "Patients with leptomeningeal metastasis are currently in a medical blind spot. They have been excluded from clinical trials involving non-small cell lung cancer, and there is a lack of research by pharmaceutical companies and medical institutions." In response to this, a team of leading hospital doctors from the Korean Society of Medical Oncology initiated a clinical trial and received approval, with Yuhan Pharmaceuticals providing the necessary medication.
Professor Kim noted, "What was surprising was that patient enrollment, which we initially thought would take two years, was completed in just one year." He added, "Patients with no treatment options for leptomeningeal metastasis view this clinical trial as a significant opportunity."
He went on to say, "There have been studies to confirm the efficacy of Leclaza in brain metastasis, but no studies have been conducted targeting leptomeningeal metastasis," adding, "As it is a researcher-initiated clinical trial, it may not directly impact the expansion of indications domestically, but some countries may refer to clinical data from South Korea when using the drug, so it is not entirely meaningless. We hope that the pharmacokinetic characteristics confirmed this time will lead to positive efficacy results in the future."

