Promising developments in anti-obesity and liver disease therapies unveiled at ICDM 2023

Il dong Pharmaceutical, widely recognized for its pioneering work on an anti-obesity drug, has unveiled its latest venture into metabolic disease research. Presenting at the International Congress of Diabetes and Metabolism (ICDM 2023) in Gyeongju, South Korea on October 19th, the "Research group on development of new drug and technology on metabolic disease" introduced Il dong Pharmaceutical's metabolic disease pipeline, which is divided into two distinct categories: metabolic diseases and liver diseases.
In the metabolic disease category, Il dong Pharmaceutical's pipeline boasts two noteworthy candidates: Xelaglifam (development code name IDG16177) and ID110521156. Xelaglifam, initially designed for type 2 diabetes, is currently undergoing phase 1 clinical trials in Germany. This drug activates the G-protein receptor 40 (GPR40) in pancreatic beta cells, stimulating insulin secretion and effectively regulating blood glucose levels.
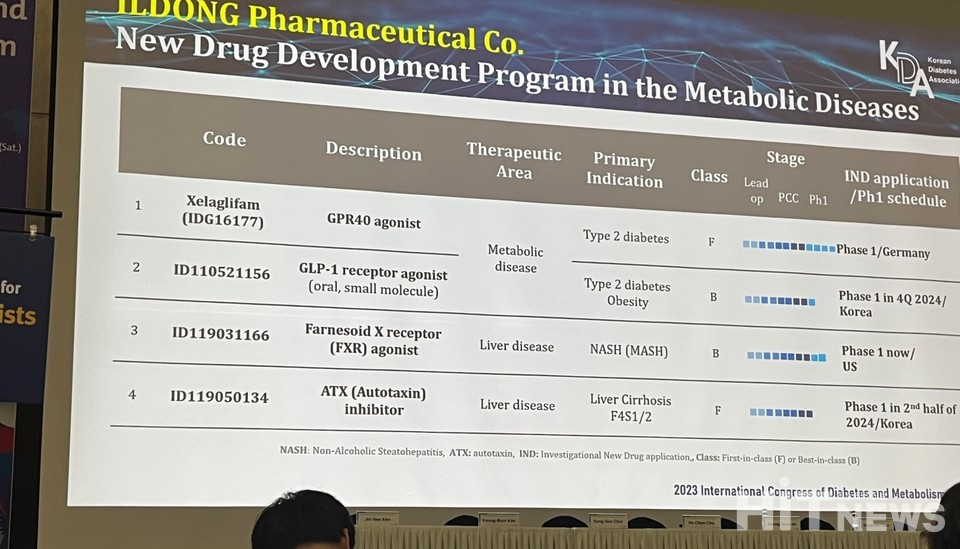
Notably, Sung-Gu Choi, the Head of Research and Development at Il dong Pharmaceutical, stated, "Xelaglifam demonstrated a significant reduction in blood glucose levels, similar to Fasiglifam, but at a dosage 30 times lower." Fasiglifam, a comparable compound developed by Takeda Pharmaceuticals, was discontinued due to concerns about liver toxicity.
ID110521156 is a GLP receptor agonist intended for the treatment of type 2 diabetes and obesity. Clinical phase 1 trials are scheduled for the fourth quarter of this year in South Korea. Il dong Pharmaceutical has recently applied for Investigational New Drug (IND) status for ID110521156 with the Korea Food and Drug Administration. The drug harnesses the power of glucagon-like peptide (GLP), known for stimulating insulin secretion and lowering blood glucose levels.
In the realm of liver diseases, the 'ID119031166' offers hope for non-alcoholic steatohepatitis (NASH). Currently undergoing phase 1 clinical trials in the United States, this therapy operates as an agonist for the Farnesoid X receptor (FXR). Director Choi elaborated, "In hamster trials, the alanine aminotransferase (ALT) levels decreased significantly, while the upregulation of FXR-targeted genes contributed to noteworthy anti-NASH effects." Elevated ALT levels are indicative of potential liver damage, as ALT is primarily found in the liver and kidneys.
Furthering their commitment to liver disease research, Il dong Pharmaceutical's 'ID119050134' targets liver cirrhosis and is poised to enter phase 1 clinical trials in South Korea next year. Liver cirrhosis, a condition characterized by fibrous tissue replacing normal liver tissue due to chronic inflammation, is known to impair liver function. ID119050134 aims to inhibit autotaxin, a protein involved in fibrotic diseases.
Notably, Il dong Pharmaceutical is in the process of establishing a subsidiary named 'Yunovia,' dedicated exclusively to research and development (R&D) efforts. Director Choi emphasized that Yunovia's primary focus will be on advancing the company's new drug development initiatives.

