Ten pioneering companies have secured certification as leaders in the innovative medical device landscape for the year 2024. According to HitNews' latest report on December 15th, Remed, Samyang Holdings, Medical IP, Quantamatrix, Recens Medical, Protia Inc, Precision Biosensor, ATsens, Emocog, and Huinno have successfully obtained certifications, marking their dedication to advancing the field of medical devices.
Certification for Innovative Medical Device Companies
The certification for innovative medical device companies, as outlined in the Medical Device Industry Cultivation and Support Act, acknowledges firms investing significantly in research, development, and production of cutting-edge medical devices in Korea. This certification is segmented into two categories: 'Innovation Leader' and 'Innovation Leap,' contingent on sales revenue and investment scale within the medical device sector.
To qualify as an Innovation Leader, companies must achieve sales surpassing $38.6 million, allocating at least 6% of that revenue to medical device research and development. Alternatively, the Innovation Leap certification is attainable for companies with sales revenue below $38.6 million, provided they invest either 8% of their revenue or a minimum of $2.3 million into medical device research and development. Notably, all ten newly certified companies have been designated as Innovation Leap recipients.
A spectrum of offerings, from surgical solutions to digital therapeutics
The ten newly certified companies showcase a diverse spectrum of innovations spanning various business domains, from digital therapeutics like Remed's electronic therapeutic solutions to Samyang Holdings' focus on surgical sutures, garnering significant attention.
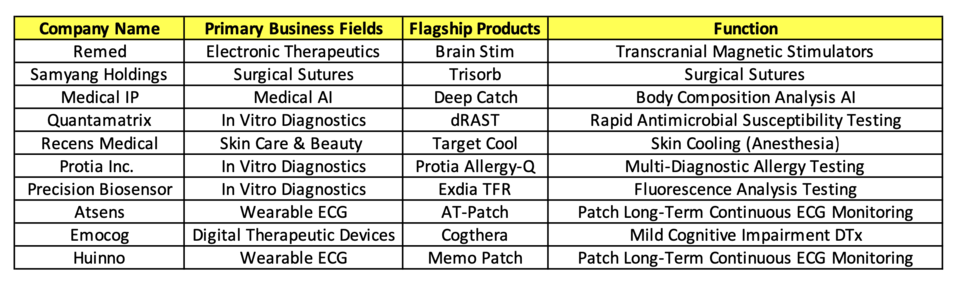
Remed specializes in 'electronic therapeutics,' specifically Transcranial Magnetic Stimulation (TMS) and Neuromagnetic Stimulation (NMS). Their flagship products include 'BrainStim' for depression treatment under TMS and 'Salus Talent Series' for chronic pain management under NMS. Samyang BioPharm, a subsidiary of Samyang Holdings, excels in pharmaceuticals and medical devices, particularly their 'Trisorb' and 'Neosorb' surgical suture series.
Among the certified companies, Medical IP operates within the digital twin framework, featuring 'DeepCatch,' an AI-based system analyzing whole-body composition via CT images. This product received certification (510(k)) from the U.S. FDA in June of the previous year.
Quantamatrix specializes in in vitro diagnostic medical devices, showcasing 'dRAST,' a rapid antibiotic susceptibility test leveraging AI deep learning for prompt and accurate antibiotic prescriptions for septicemia patients, omitting the need for separate culture processes.
Recens Medical focuses on 'rapid precision cooling' technology for medical devices like ophthalmic cooling anesthesia equipment and precision cooling therapy devices, notably their product 'Target Cool,' which induces instant anesthesia effects by cooling the skin.

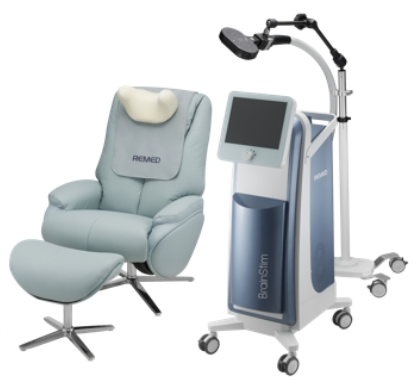
Protia presents 'Protia Allergy-Q,' a multi-diagnostic testing device for allergies, accompanied by related patents and supply commencement to Lal Pathlabs, India's largest diagnostic testing institution.
Precision Biosensor excels in in vitro diagnostic devices with 'Exdia TFR,' utilizing fluorescence analysis testing technology for both human and animal use.
ATsens specializes in wearable monitoring medical devices, offering 'AT-Patch,' a long-term ECG monitoring device surpassing traditional Holter monitoring limitations, gaining increased collaboration within the pharmaceutical sphere.

Huinno focuses on wearable biosignal measurement technology, featuring 'Memo Patch' and 'Memo Patch Plus,' which offer ECG monitoring for up to 14 days and have secured joint promotion contracts with J&J MedTech Korea and Yuhan Pharmaceuticals.
Emocog stands as a digital healthcare company with 'Cogthera,' a digital therapeutic device catering to mild cognitive impairment (MCI), providing personalized cognitive intervention therapy and recognized as Innovative Medical Device No. 35 by the MFDS in June 2023.

